Biomass Valorization
Здесь есть возможность читать онлайн «Biomass Valorization» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomass Valorization
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Biomass Valorization: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomass Valorization»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the potential of biomass-based chemicals with this comprehensive new reference from leading voices in the field Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization: Sustainable Methods for the Production of Chemicals
Biomass Valorization — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomass Valorization», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
1.3 Lignocellulosic Biomass
Of all types of biomass, lignocelluloses are the most available on the planet, ranging from wood and forestry waste to straw and agricultural waste. Lignocellulosic biomass is composed of cellulose (40–50%), hemicellulose (15–20%), lignin (25–35%), and other elements ( Figure 1.1). Both cellulose and hemicellulose are carbohydrate‐based polymers, while lignin is an aromatic polymer. Cellulose is a linear, glucose‐based polymer, making it a good source of this C6‐sugar. Cellulose cross‐links with hemicellulose, a branched polymer composed of different C5‐carbohydrates, uronic acids, and C6‐sugars. Lignin, perhaps the most irregular component of lignocellulose, is a polyaromatic macromolecule composed of phenylpropane derivatives. Lignin is mostly responsible for structural rigidity within the lignocellulose. Further, lignocellulosic bio‐feedstocks include variable quantities of pigments; terpenes; inorganic elements such as Mn, K, P, Cl, Ca, Mg, and Na, as well as Al, C, Fe, N, S, Si, and Ti to a smaller extent; and various extractives, e.g. carbohydrates, proteins, lipids, waxes, chlorophyll, terpenes, tannins, and uronic acids.
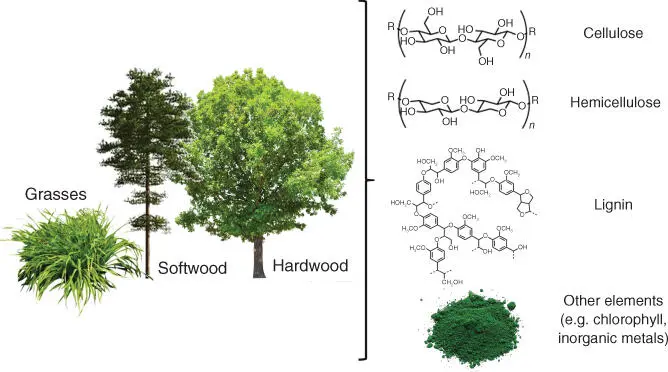
Figure 1.1 Schematic representation of the components of lignocelluloses.
An extensive and systematic review on the composition of various types of biomass shows the significant changes in the composition of these elements depending on the type of biomass [41].
Overall, lignocelluloses are made of highly oxygenated C5‐ and C6‐derivatives. The oxygen functionalities make lignocelluloses a much different feedstock to petroleum sources that are mainly hydrocarbons. The oxygen functionalities in lignocelluloses are in some cases advantageous because they can minimize oxidation reactions, which usually have a negative environmental impact, and favor reduction reactions, which are typically milder processes and have less environmental impact. Further, the propensity to produce coke/humins and ash obliges the use of mild temperatures for these by‐products' minimization, as opposed to the traditional catalytic cracking/reforming of fossils. In fact, the presence of plenty of oxygen functionalities and low volatility tend to lead to the molecules' decomposition at high temperatures, generating carbonaceous residues.
Lignocelluloses have variable composition in their singular components depending on the plant origin. Water and inorganic residue contents also vary significantly from grass to wood. Although composition does vary significantly, biomass can source several useful compounds, including carbohydrates, aromatics, terpene, and fatty esters. These different components can be isolated and converted for use in many applications including pharmaceutical, cosmetics/perfumes, plastics, textiles, and specialty chemicals. For this, several different biomass valorization routes can be envisioned with a wide range of obtainable products.
1.4 Key Biomolecules
During the first attempts of biomass valorization, drop‐in energy solutions have been investigated as they could directly substitute the use of fossil resources for transportation vehicles. The most common examples are the use of bioethanol and biodiesel as additives to common automotive fuels. Bioethanol is mostly produced in industry using yeast fermentation of C6‐sugars. With an increase of 25 billion gallons (roughly 75 Mt) worldwide, bioethanol is one of the most mass‐produced bio‐based molecules. However, starchy feedstocks (i.e. first generation) are mostly used in the production of bioethanol, causing direct competition with the food market, widespread deforestation, and concerns on the presence of enough food sources for both humans and animals [42]. Also, bioethanol has limited competitiveness with petroleum options because of low product value and relatively high price, especially when considering food sustainability. To add perspective, the price of oil would have to be above $70–80 per barrel for bioethanol to be competitive from a cost standpoint, while today, oil is at <$40 per barrel [43].
Alternatively, another approach is to obtain different platform molecules from biomass that can be used for production of a wide variety of chemicals. With a shift on how we perceive platform molecules, new chemical (and biological) pathways can be envisioned. In order to induce this shift, several important bio‐products unique from petrol‐based ones were identified in a 2004 US Department of Energy report [44]later updated by Bozell and Petersen [45]and further reviewed by Gallezot [46]. Bio‐based platform chemical families and their respective processes, industrial applications and current technical challenges, are summarized in Table 1.1 [ 44– 47]. For more information on the industrial challenges for biomass valorization, the reader is referred to Chapter 13 of this book.
Table 1.1 represents only a small fraction of all the molecules that could be identified as valuable platform chemicals, opening a significant number of possibilities for the synthesis of petrol‐like or new molecules. However, apart from established processes such as those of sorbitol and glycerol, all other biomolecules generally suffer from high production costs that might be caused by
1 high price of feedstocks (depending on the required sugar purity).
2 low resource efficiency (e.g. synthesis of by‐products that lower conversions and intensify purification/separation processes).
3 high investment and operational cost required for the reactor volumes or design, or need to maintain sterile conditions during production.
4 inefficient catalysts, which could be (a) biological (enzyme and bacteria), which require metabolic engineering for higher efficiency and durability; (b) homogeneous, which tend to be corrosive, toxic, or difficult to reuse and recycle; or (c) heterogeneous, which have lower conversions even if they can be recovered and reused, but are prone to irreversible adsorption of organic molecules, leading to coke and thus reactor fouling.
Particularly, when compared to petrol‐like compounds, the disadvantages of chemicals from biomass processing become increasingly apparent in terms of overall costs. Even when only considering feedstock transportation, the advantage goes to petroleum, as it is a fluid that can be pumped (or natural gas through pipelines). Biomass tends to occupy larger volumes, given its physical nature, and is much more difficult to transport as a result. Nevertheless, the most notable difference that gives petrol‐like compounds the slight edge is the absence of oxygen functionalities (aliphatics/aromatics/olefins), which reduces their reactivity but yields larger production volumes by the addition of heteroatoms. In fact, although fossil compounds are modified via oxidation, bio‐derived compounds often require oxygen removal. In this sense, larger initial volumes are required for biomass to reach the same final product volume, making it economically inefficient. Moreover, the reactivity of oxygen groups in biomass gives inefficient processes, especially if targeting petrol‐like compounds. In this regard, a better route is to build off these different functionalities and explore new platform chemicals that are specific for biomass products. Most of the advances have been achieved largely because of catalytic pathways that allow for lower energy requirements and higher resource efficiency.
Table 1.1Key examples of the possible bio‐based products, state‐of‐the‐art processes, and challenges [ 44– 47].
| Bio‐product platform (example) | Process | Industrial application | Technological challenge |
|---|---|---|---|
| 1,4‐Diacid (succinic acid) | Anaerobic fermentation | Pharmaceutical, food, polymers, solvents | Separation/purification of products |
| Furanics (HMF) | Acid‐catalyzed dehydration of C‐5 and C‐6 sugars/oxidation | Food/cosmetics, polymers, construction, textiles, fuels | Low resource efficiency |
| 3‐Hydroxypropionic acid (acrylic acid) | Aerobic fermentation | Polymers, textiles | Low resource efficiency |
| Under metabolic engineering research | |||
| Amino acid (aspartic and glutamic acids) | Microbial process | Biodegradable polymers, pharmaceuticals | Need of sterile conditions |
| Complex separation | |||
| Under metabolic engineering research | |||
| Gluconic acid (methylglucoside) | Aerobic fermentation/catalytic oxidation | Food, pharmaceuticals | Low resource efficiency/catalyst deactivation |
| Itaconic acid (itaconic anhydride) | Aerobic fermentation | Specialty polymers (including biodegradable) | Low resource efficiency |
| Under metabolic engineering research | |||
| Glycerol (dihydroxyacetone) | By‐product of biodiesel/soap manufacture | Cosmetics, food, pharmaceuticals, lubricants, polymers, Li batteries | Low market price |
| Expensive purification | |||
| Catalyst separation/deactivation in upgrade | |||
| Levulinates (γ‐valerolactone) | Acid‐catalyzed dehydration of C‐6 sugars | Fragrances, food, fuels, solvents, pharmaceuticals, polymers | Low resource efficiency |
| Sorbitol (isosorbide) | Hydrogenation of C‐6 sugars | Food, pharmaceuticals | Established technology |
| Low market price | |||
| Lactones (3‐hydroxybutyrolactone) | Oxidative degradation of C‐5 and C‐6 sugars | Pharmaceuticals, chiral building block, polymers | Inefficient oxidation |
| Low resource efficiency unless starch is used | |||
| Inhibitory effect of biomass | |||
| Lactic acid (oxalic acid) | Anaerobic fermentation | Cosmetics, pharmaceutical, biodegradable polymers | High feedstock cost (high‐purity lignocellulosic sugars or food derived) |
| Separation/purification of products | |||
| Biohydrocarbons (isoprene) | Aerobic fermentation | Polymers | Investment cost (reactors) |
| High feedstock cost (high‐purity lignocellulosic sugars or food derived) | |||
| ABE (acetone, butanol, ethanol) | ABE fermentation | Fuels, solvents | High feedstock cost |
| Low resource efficiency | |||
| Lignin | Catalytic decomposition | Polymers, food, pharmaceuticals, fuels | Low resource efficiency |
Sources: Werpy et al. [44], Bozell et al. [45], Gallezot [46], Isikgor et al. [47].
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomass Valorization»
Представляем Вашему вниманию похожие книги на «Biomass Valorization» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomass Valorization» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












