Mohamed N. Rahaman - Materials for Biomedical Engineering
Здесь есть возможность читать онлайн «Mohamed N. Rahaman - Materials for Biomedical Engineering» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Materials for Biomedical Engineering
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Materials for Biomedical Engineering: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Materials for Biomedical Engineering»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A comprehensive yet accessible introductory textbook designed for one-semester courses in biomaterials Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering: Fundamentals and Applications
Materials for Biomedical Engineering — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Materials for Biomedical Engineering», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Until recently, stent designs focused strongly on the need to provide a mechanical function. This called for the use of a biocompatible metal with the attractive mechanical properties characteristic of this class of materials and corrosion resistance to minimize the chances of rejection in vivo . Early stent designs employed durable metals that were available off the shelf, such as a stainless steel or a cobalt–chromium (Co–Cr) alloy ( Figure 1.4). Although these so‐called bare‐metal stents work well initially, they themselves can become blocked after a few years (a process referred to as restenosis). A problem with bare‐metal stents is that they can irritate the endothelial lining of the artery. This triggers an inflammatory response, which leads to the proliferation of certain cells (vascular smooth muscle cells) and the growth of extracellular fibrotic tissue into the lumen of the stent, resulting in restenosis. The result is that the procedure may have to be repeated at regular intervals.
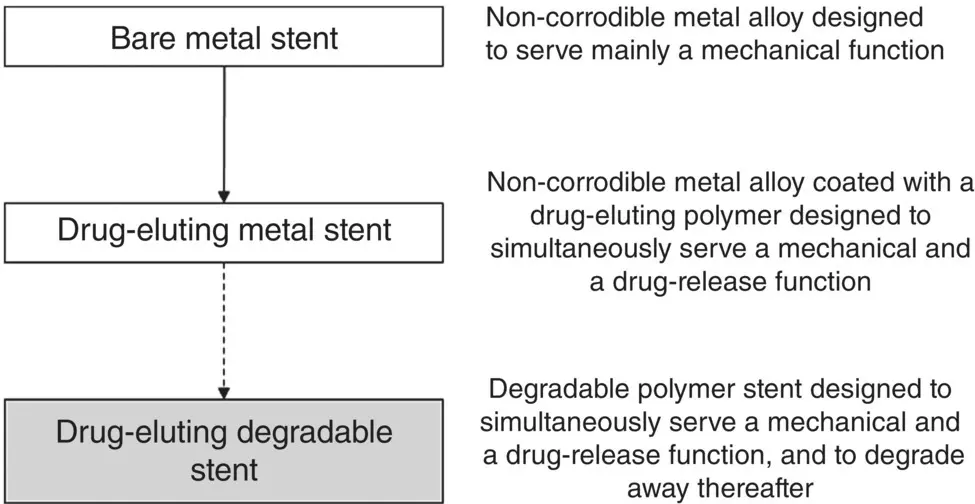
Figure 1.4 Stages in the evolution of the intravascular stent, used as an example to illustrate the considerable shift in biomaterials design over the last few decades.
Modern metal stents are designed to serve the mechanical function of bare‐metal stents but to also provide a biological function to reduce restenosis. The biological function of controlling cell proliferation and the growth of fibrotic tissue into the stent is achieved by incorporating a drug‐eluting function into the stent. In one approach, a thin coating of a polymer, degradable or nondegradable, in which the drug is dispersed, is applied to the stent surface. Commonly used drugs include paclitaxel and sirolimus, both of which are powerful antiproliferative drugs that inhibit cell proliferation. The use of these drug‐eluting stents has produced a significant lowering of restenosis rates, but they have not been entirely successful. A problem is that the stent can provide sites for aggregation of platelets (from the blood), resulting in thrombosis, which normally necessitates prolonged antiplatelet therapy.
Questions have been raised about the need for the continued presence of the stent after stenosis has been resolved. It has been claimed that the benefit of the drug‐eluting stent is achieved within a finite time, such as a few months, and the continued presence of the stent contributes to adverse biological effects due to its ability to irritate the endothelium of the artery. Consequently, a more recent approach to the design of modern stents is the use of a degradable stent, composed of a degradable polymer in which the drug is dispersed or a degradable metal coated with a degradable polymer in which the drug is dispersed. The stent serves a mechanical function initially to open up the blocked blood vessel and, as it degrades, the drug is released at a controllable rate to destroy the deposit responsible for stenosis. After resolution of stenosis, the stent degrades completely. While drug‐eluting degradable stents provide a useful alternative approach to treating stenosis; a few questions related to their effectiveness remain unresolved at the present time. One is the degree of inflammatory response by cells to the degradation products of the polymer stent and the effect of this response on the endothelium of the blood vessel.
1.3.2 Factors in Biomaterials Design and Selection
The design of a biomaterial for a specific application starts with the selection of a material with certain inherent properties. Then materials science, biological and engineering principles and procedures are utilized to design and create an implant with the requisite properties for the intended application. Important factors that should be considered in biomaterials design and selection are summarized in the following sections.
Chemical composition: It is well known in materials science that the chemical composition (or atomic structure) of a material controls its intrinsic properties, such as mechanical strength and stiffness, whether the material is brittle or not, its ability to degrade in an aqueous environment, whether the material is electrically conducting or not, and so on. Consequently, the chemical composition is perhaps the first factor to be considered in selecting a material for use as a biomaterial.
Biocompatibility: The ability of a material to perform with an appropriate host response in the intended application and its continued function is a critical factor in the selection of a biomaterial. Biocompatibility, as described earlier, is a property of both the biomaterial and the tissue system in which it is implanted, or with which it interfaces. The biomaterial should not, for example, be toxic or release ions or molecules that are toxic locally or systemically.
Mechanical properties: All tissues (and organs) in the body have some characteristic mechanical properties, such as strength and stiffness. Consequently, the mechanical properties of a biomaterial are important in determining its performance in vivo. A useful guideline is that a biomaterial used to repair a diseased or damaged tissue should have mechanical properties that are comparable to the host tissue. However, this is often difficult to achieve. A large mismatch in mechanical properties between an implant and the host tissue can lead to adverse biological effects. The use of a strong metal implant to repair a bone defect can, for example, lead to resorption and weakening of the surrounding bone and, eventually, to bone fracture. The mechanical properties of a material can also influence the response of cells and, thus, they can determine the ability to regenerate a specific tissue. For example, a material that is optimal for regenerating bone typically would not be suitable for regenerating a soft tissue such as cartilage.
Stability in the biological environment: A biomaterial should be nondegradable or should degrade at a desirable rate for the intended application. For a degradable biomaterial, a guideline often found in the literature is that the implant should degrade at a rate comparable to the rate at which new tissue is being formed. However, this can often be difficult to achieve in practice.
Ease of fabrication: Typically, a biomaterial will have an external shape (or geometry), complex or simple, that is similar to the tissue or organ to be replaced or regenerated. Biomaterials can be 3D objects, fibers, coatings, films, or particles, depending on the application. The biomaterial might be required to have internal structural features that are also important. These internal structural features relate to the way in which the components or phases, such as the solid phase and porosity, are arranged within the biomaterial. The structure at a microscale and nanoscale, referred to as the “microstructure” and “nanostructure,” respectively, are often the major structural features of interest. The microstructure (or nanostructure) can be simple or complex, depending on the application. Materials used to create biomaterials should be capable of being formed economically into the desired external shape and internal microstructure (and/or nanostructure). In common with other technologies, the use of additive manufacturing to create biomaterials, particularly with complex shape and microstructure, has been increasing rapidly.
Ability to be sterilized: A biomaterial should be capable of being sterilized by one of the sterilization methods that employ heat (steam autoclaving or dry heat), gas, radiation, or electron beam treatment prior to their use in studies in vitro or implantation in vivo. Lack of sterility will invariably lead to infection and destruction of cells, followed by potentially catastrophic failure in vivo.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Materials for Biomedical Engineering»
Представляем Вашему вниманию похожие книги на «Materials for Biomedical Engineering» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Materials for Biomedical Engineering» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












