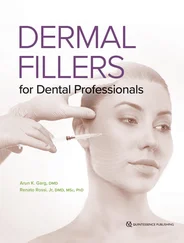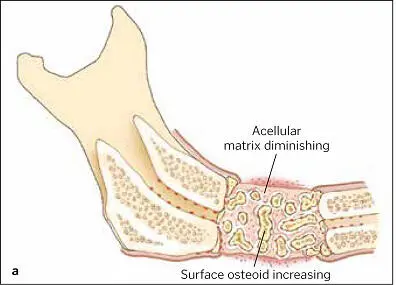
FIG 1-15 (a) By fusing graft particles together and to the host bone, the graft has produced sufficient osteoid to consolidate by 6 weeks. (b) Corresponding radiograph shows condensation of the cloudy graft appearance, indicative of osteoid production and graft organization. The radiolucent line between the graft and host bone has nearly disappeared as a result of osteoconduction between the graft and host bone edge. (Reprinted with permission from Marx and Garg. 1)

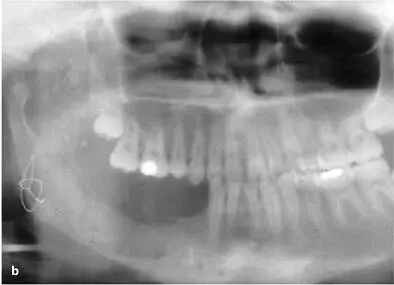
FIG 1-16 (a and b) At about 6 weeks, the graft begins a major resorption-remodeling cycle in which osteoclasts resorb the disorganized immature bone and release BMP and insulinlike growth factors, thus inducing formation of new bone that will mature during function. (Reprinted with permission from Marx and Garg. 1)
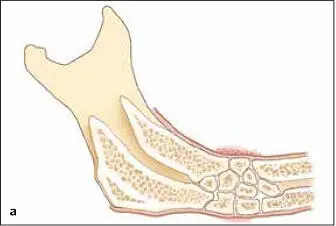
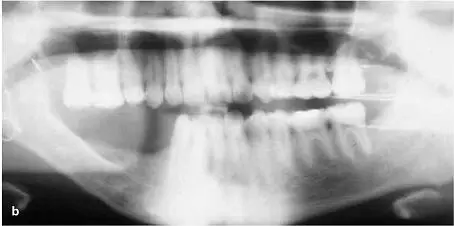
FIG 1-17 (a) After 6 weeks, the graft will be consolidated and fused to the host bone. It then enters the lifelong resorption-remodeling cycle of the remainder of the skeleton. (b) Radiographically, bone maturation is characterized by the development of a normal trabecular pattern and an increased density. Here, an inferior border outline, an external oblique ridge, and a coronoid process attest to the remodeling of bone under function. (Reprinted with permission from Marx and Garg. 1)
Platelet growth factors not only induce bone cell regeneration but also double the normal increase in mineral density in bone, with faster-forming and more quickly maturing bone, including significantly increased trabecular bone values 48( Fig 1-18). Bone-related therapies for PRP include oral and cranial surgery, 48,122–124spinal fusion, 125–128osteogenesis distraction, 129–131foot and ankle fractures, 132–134bone grafting, 135,136oral implants, 137–143and diabetic fractures. 78,144,145
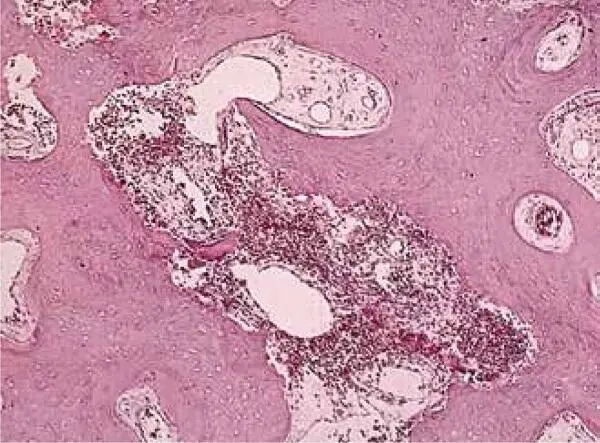
FIG 1-18Histomorphometry of an autogenous bone graft without PRP at 4 months shows that the graft has a 60% trabecular bone density, consists mostly of immature bone, and is undergoing active resorption-remodeling. (Reprinted with permission from Marx and Garg. 1)
Efforts to standardize protocols for the clinical application of autologous platelet-derived product have often been accompanied by nonstandardized nomenclature for that product. Many researchers believe the term platelet-rich plasma is too general and incomplete, leading to confusion not only in preparation and application but also in cataloging in the scientific databases, thus hampering the flow of information in the scientific community. Other names that have been applied over the years include platelet-rich fibrin (PRF), autologous platelet-rich plasma (aPRP), platelet gel (PG), autologous platelet concentrates (APCs), preparation rich in growth factors (PRGF), platelet-derived growth factor (PDGF), platelet-leukocyte gel (PLG), concentrated growth factors (CGFs), and numerous others ( Fig 1-19).

FIG 1-19The terminology for PRP is a confusing alphabet soup of acronyms. Chapter 4presents the names and formulations for eight configurations of PRP and the step-by-step instructions for preparing them.
A simplified terminology-classification system for platelet concentrates has been widely adopted. It consists of eight categories based on its consistency and clinical application. The researchers and clinicians who devised this classification system believe it underscores the profound potential of these products and the need for all stakeholders to learn and demonstrate their awareness of the products’ complexity. The composition and application of these other forms of PRP are detailed in the chapters of this book.
Historically, the majority of studies of the clinical efficacy of PRP did not provide sufficient information to allow their PRP preparation protocol to be reproduced. This not only added to the confusion over terminology but also made it impossible to compare the PRP products that were being tested. Several recent papers have addressed this confusion and shared ideas about how to standardize the protocol to make PRP preparation simple and straightforward, and most importantly, to obtain a consistent and effective platelet yield. 146–148In 2017, Chahla et al published a systematic review of the clinical orthopedic literature on PRP and a call for more standardized reporting of PRP preparation protocols and composition. 146Although 105 studies met the inclusion criteria for analysis, only 11 of them (10%) provided sufficient detail of their preparation protocol to allow it to be reproduced. Additionally, only 17 studies (16%) provided quantitative metrics on the composition of the PRP product they delivered to patients.
Since the mid-1990s, clinicians have used an assortment of PRP preparations in several different therapies, ranging from oral and maxillofacial surgery and implantology to orthopedic surgery, burn treatment and the treatment of other wounds difficult to heal, soft tissue cosmetic surgery, soft tissue disease and injuries, and tissue engineering. Invariably, the literature that documents these therapies calls not only for high-level studies to better measure the efficacy of the variety of PRP therapies but also for standardizing the preparation and application of platelet-derived products. 1–4
Any discussion of PRP application must be preceded by a review of platelet sequestration principles. Only then can a discussion of progress toward consensus on PRP protocols take place. Such an approach reveals that the clinical preparation and application of PRP across medical disciplines is a dynamic, evolving process, and that we disrupt this dynamism at our medical/scientific peril. Too-hasty attempts to reach consensus could inhibit researchers’ efforts to discover the full potential of PRP and other biologic therapies.
Naming and classifying platelet derivatives
Nearly 25 years after the earliest reports appeared, the literature on PRP for both human and veterinary medicine still lacks consensus on standardized protocols for preparing, naming, classifying, and applying platelet concentrates. PRP appears to remain the most likely term, given that plasma resuspension of any platelet concentrate is required before application. 1A high-density fibrin concentrate can facilitate cell migration and the release of cytokines. However, the number of leukocytes affects the concentrate’s wound healing ability, leading to the often contradictory results of different studies.
Читать дальше