In the mid-1980s, platelets were understood essentially as cells that helped to stop bleeding. Over the next 20 years, the discovery of the various growth factors released by platelets gave birth to regenerative medical therapies, most of which are still in their infancy. 24–35How the growth factors and functional matrix delivered by autologous blood concentrates induce wound healing is widely understood. The focus of current research is replicating and standardizing the preparation and administration of the autologous-derived product to best suit the donor-patient. Though a variety of preparation techniques, products, and nomenclatures have been tried, the good news is that no significant difference in the osteogenesis of growth factors has been evidenced. 36–40
Nevertheless, firmly establishing the science of platelet-rich plasma and other platelet-derived products requires an investigation of platelet biology, the release of growth factors, and the practical application for soft tissue healing and bone tissue regeneration. So far, the scientific journey of autologous blood concentrates has been remarkably expansive. The future of this journey promises to be more focused, even single-minded, toward its scientific destination—even more standardized products and procedures based on replicable results.
The first autologous blood concentrate was introduced in the literature as autologous fibrin adhesive and later changed to platelet-rich plasma (PRP). That term became standard, first in the oral surgery literature and then in all medical and dental surgical specialties. While many other terms have been used to describe autologous blood concentrates—particularly in niche markets as a way to sell specific centrifuges and/or test tubes—PRP will be used throughout this book.
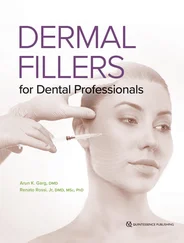
As an actor in the performance of regenerative medicine, 41–47PRP provides two of the three essential components for allowing a wound to heal in place: growth factors and a scaffolding stage ( Fig 1-1). The third ingredient for in situ tissue regeneration is the cells. PRP is a patient’s own blood concentrate, modified in a relatively quick, efficient, safe, and simple procedure, to obtain a dense concentration of platelets. The autologous nature of PRP precludes disease transmission to the patient or other adverse reactions. To provide wound healing benefit to the patient, PRP generally must have at least four to seven times the normal concentration of platelets, or roughly 1 million platelets per microliter. 48A blood clot in a wound consists mostly of red blood cells and much smaller percentages of platelets and white blood cells ( Fig 1-2). Applying PRP to such a wound essentially replaces red blood cells with growth factor–producing platelets and a fibrin network, thus (at least in theory) greatly enhancing the healing of wounds and migration of cells, as well as the regeneration of bone and soft tissue.
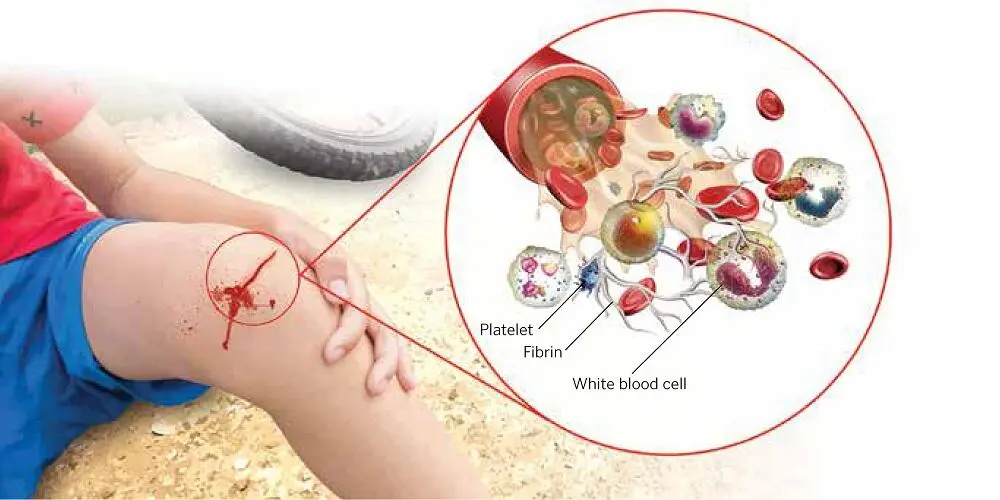
FIG 1-1Components of blood that are concentrated in PRP.
When bone marrow megakaryocytes undergo cytoplasmic fragmentation, the anuclear platelet cells enter the circulatory system. The relatively tiny platelet is about one-fourth the size of a red blood cell (approximately 8 µm in diameter) and six to seven times smaller than a lymphocyte; however, the platelet’s membrane extends pseudopodially via invaginations, which provide an expansive, dynamic, and vigorous surface area for the cell membrane during activation. 1,49The plasticity and resilience of the platelet’s pseudopodic membrane enable its vascular-sealing qualities, along with its ability to form a thrombus and fibrin clot, as well as clot retraction when its hemostatic labors are complete 50( Fig 1-3). Generally, the larger and younger the platelet, the greater its hemostatic qualities and the greater the quantities of growth factors contained within it. 23,51,52
The short lives of platelets (240 hours or less) are very actively spent synthesizing and secreting growth factors as part of the blood-clotting process. The platelet contains lysosomes, ribosomes, mitochondria, and an assortment of intercellular proteins that help form its shape as well as its mobility. The platelet cell also contains storage organelles that consist of lysosomal granules (for storing enzymes for digestion), dense granules (for storing and secreting adenosine diphosphate [ADP]), and alpha granules (for summoning and activating other platelets via nascent growth factors) ( Fig 1-4). 53–55
The growth factors stored in the alpha granules include platelet-derived growth factor (PDGF) isomers labeled AA, BB, and AB (referred to as polypeptide “dimers” because of their two active sites, which are actually antiparallel monomers); transforming growth factor (TGF) isomers beta 1 and 2; vascular endothelial growth factor (VEGF); and epithelial growth factor (EGF). Growth factors not contained in platelets include insulinlike growth factors (IGF) 1 and 2 and bone morphogenetic protein (BMP). The blood-clotting process activates the alpha granules in platelets to secrete growth factors, both when the platelets circulate normally in the blood and when the platelets are concentrated in PRP. Alpha granules move toward the membrane and bind themselves to its surface, causing histone and carbohydrate side chains to combine with, and to activate, growth factor proteins. 1
FIG 1-2The classic wound healing cascade.
In addition to their basic hemostatic roles, platelets have been found to play a number of nonhemostatic functions. 56,57Tissue repair and inflammation are two of several functions that researchers are currently exploring. 58,59The alpha granules in platelets are the producers/directors of the diverse roles medical science is learning platelets can play beyond the traditional role of hemostasis. Ironically, in fact, the granules contain substances that normally work in opposition to each other. The platelet’s ability (when properly signaled) to release different substances specifically required by other cells, molecules, and tissues explains why it is the focus of so many different types of current medical research, including tissue inflammation and regeneration, neurology, autoimmunity, hemostasis, wound healing, atherosclerosis, and a diverse range of others. 60–66
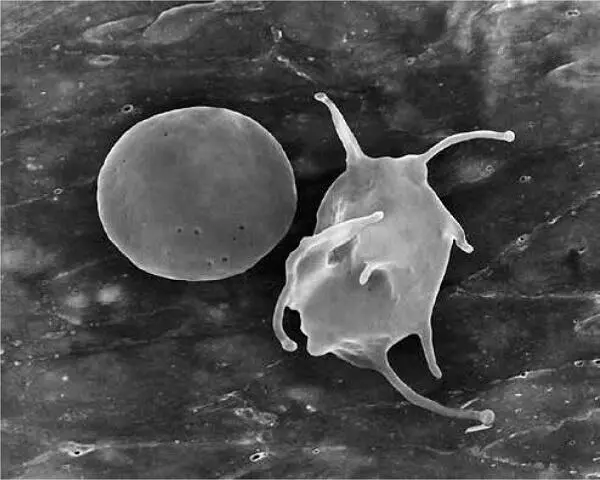
FIG 1-3Scanning electron micrograph (SEM) appearance of a platelet before (left) and after (right) activation.
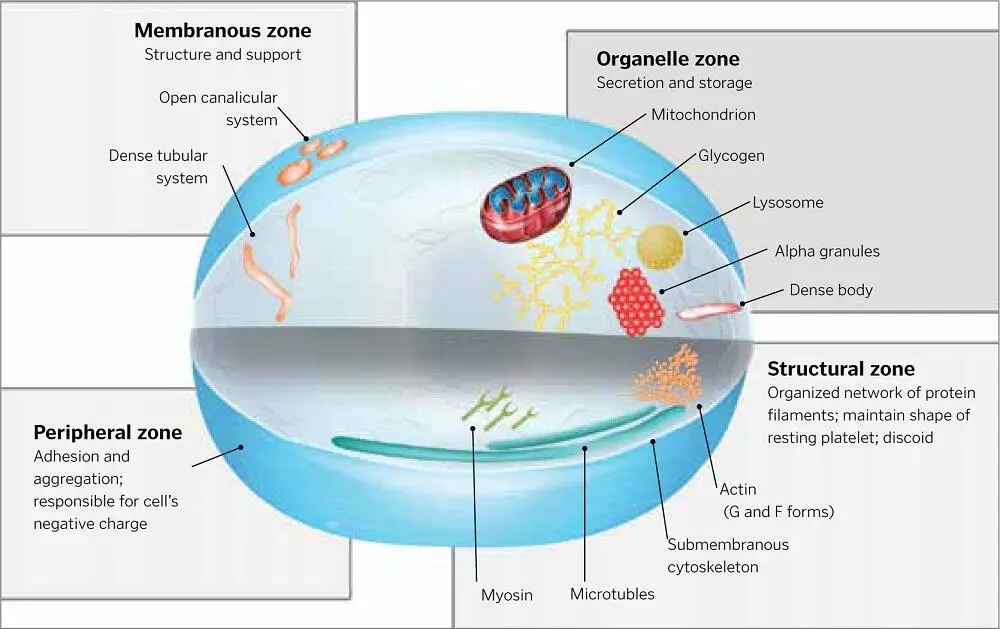
FIG 1-4Shape and functions of platelet cells.
There are a number of different types of growth factors contained in platelets. 67–69These growth factors are polypeptides, accounting not only for tissue and organ morphogenesis (from shortly after human conception to adulthood) but also for cell differentiation and proliferation. Their crucial activity in cell healing makes them especially important to current research in tissue engineering and regenerative medicine ( Fig 1-5). The growth factors that have received the most attention from medical researchers and practitioners include PDGF-AA, PDGF-BB, and PDGF-AB; TGF-β1, TGF-β2, and TGF-β3; fibroblastic growth factor (FGF); IGF; EGF; and VEGF.
Читать дальше