PRP and Soft Tissue Healing
PRP has shown great promise clinically and histologically, especially for healing soft tissue in a standard wound of a donor site for a split-thickness skin graft. 101,102Additionally, healing therapies for burns may benefit significantly from PRP application. The platelets release growth factors and cell-signaling cytokines, such as interleukin and interferon, that act to regulate inflammation and infection in the immune system 103( Fig 1-11).
When compared to non-PRP–assisted clotting, PRP-assisted clotting is remarkable for its rapidity of healing in the basal cells at the edge of the wound, where EGF induces epithelial proliferation; subsequent migration to the granulation tissue helps the clot’s cell adhesion molecules. Unlike an unassisted clot, the PRP clot reveals the bundles of fibroblasts and collagen, evidence of an expanding epithelium, and more mature healing. This comparatively accelerated maturity is also evidenced by increasingly reduced vascularity and fibroblastic cellularity over time, as well as quicker fleshlike appearance in 2 to 6 months. Reduced pain in the first 7 days of the wound, and reduced scarring over time, are also notable differences effected by the PRP clot. These benefits are also demonstrated in healing of other soft tissue wounds, including mucosal flaps, dermal fat grafts, and similar wounds. 1
Bone and soft tissue healing can be strengthened in a variety of surgical procedures when a concentrated mixture of autologous platelets is placed at the wound site. The relative ease of methods for obtaining PRP makes it an attractive regenerative adjunct therapy for many surgical treatments. Promoters of PRP tout its ability not only to help restore damaged bone and soft tissue but also to enhance wound healing, lower the patient’s pain and discomfort after the surgical procedure, and reduce the rates of infection and loss of blood. 104–106Much of the recent literature on PRP has been devoted to its wide range of applications in tissue healing and repair, including maxillofacial, periodontal, oral ( Fig 1-12), and plastic surgery; heart and spine surgery; and chronic ulcers of the skin and soft tissue. 107–110Some studies have suggested that in addition to enhancing wound healing, PRP provides antimicrobial qualities to inhibit postoperative infections in oral surgery. 111PRP has been used in such soft tissue therapies as ligament, muscle, and tendon repair 38,112; rotator cuff tears 113–115; skin ulcers 116,117; acne scarring 118; and limb amputation. 119–121
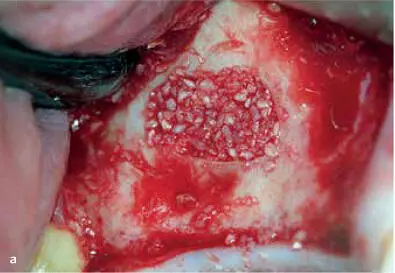
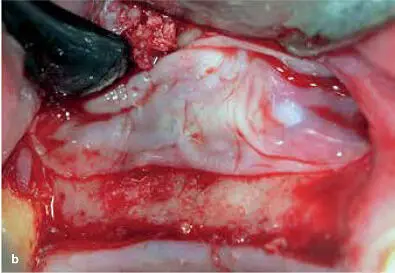
FIG 1-12Application of PRP in a sinus elevation procedure. (a) Once an adequate volume of graft material hydrated with PRP liquid has been placed, the window is ready for a PRP membrane. (b) The PRP membrane is placed over the lateral window, and then the flap is sutured back in place.
PRP and Bone Regeneration
PRP accelerates and expands cells’ wound healing response and acts biochemically to set the rate and amount of regeneration in bone. Within 10 days, its activity is complete, but this short action has long-lasting effects. For example, the alpha granules in platelets degranulate within several minutes of clot formation, and within 1 hour 90% of their growth factors are released, stimulating osteoprogenitor, endothelial, and mesenchymal stem cells. A graft-surrounding matrix is formed by fibrin, fibronectin, and vitronectin. PDGFs have a mitogenic effect on osteoblast, endothelial, and mesenchymal stem cells. The latter are also acted upon mitogenetically and angiogenetically by TGF-β isomers, which induce osteoblastic differentiation as well. While capillary ingrowth is promoted by VEGF, the lack of epithelial cells renders EGF inert ( Fig 1-13a). Within about 72 hours, osteoprogenitor cell mitosis begins and capillary buds appear ( Fig 1-13b). In the entire first phase of bone graft healing (about 2½ to 3 weeks), the graft is penetrated by capillaries, and osteoprogenitor cells have greatly proliferated ( Fig 1-13c). During this phase, cell instability and infection are common, with the potential for lysing and arresting the development of wound healing. Obviously, prevention of infection and contamination are essential, as is graft stability. 1
The hypoxic and acidic atmosphere of the wound itself attracts the circulating macrophage and blood monocyte (soon a wound macrophage), both of which assist bone regeneration via the secretion of more growth factors. The clot now contains fibrin, fibronectin, and vitronectin, acting as a matrix for the ingrowth of blood vessels as well as the proliferation and migration of cells. Between 3 and 6 weeks, the proliferation and differentiation of osteoprogenitor cells in the matrix produce osteoid ( Fig 1-14), which signals the next (second) phase of healing, when graft and bone join and when adventitial cells develop to support the vascular ingrowth ( Fig 1-15). Hypoxia diminishes due to the oxygen provided by the increased blood flow, preventing hyperplasia. By week 6, osteoclasts resorb the osteoid, releasing BMPs and IGF factors 1 and 2, causing the differentiation of nearby osteoblasts and mesenchymal stem cells for maturing bone replacement ( Fig 1-16). Mineralized dense bone thus becomes the normal formation now, in the third phase of bone regeneration, as the graft-fused bone life cycle parallels the regular turnover rate of bone replacement in the body ( Fig 1-17).
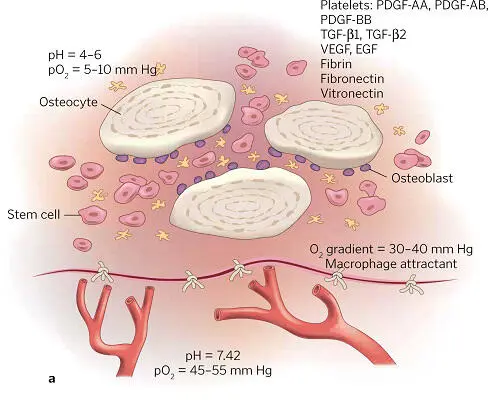
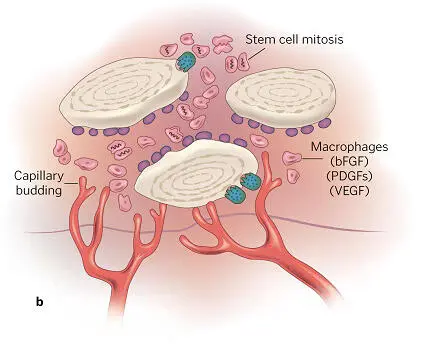
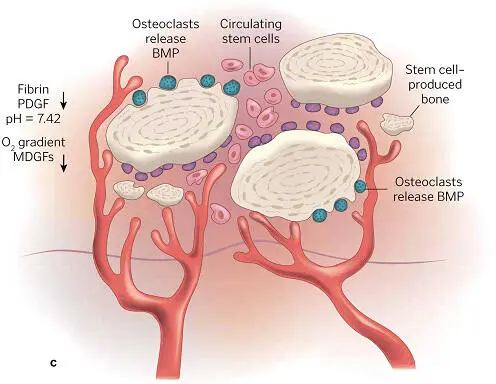
FIG 1-13 (a) The biochemical environment of an autogenous bone graft. (b) As early as 3 days after graft placement, significant cell divisions and penetration of capillary buds into the graft can be seen. (c) By 17 to 20 days, complete capillary penetration and profusion of the graft has taken place, and osteoid production has been initiated. (Reprinted with permission from Marx and Garg. 1)
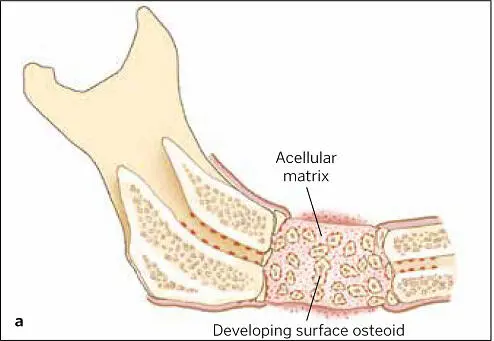
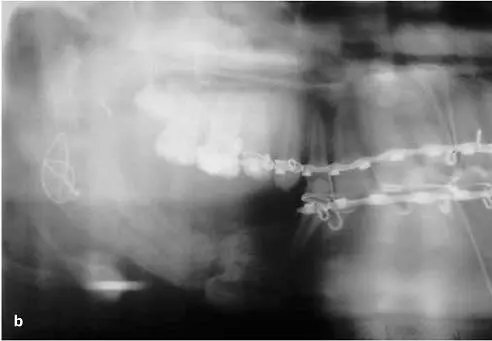
FIG 1-14 (a) Acellular matrix along with surface osteoid developing on the endosteal surfaces of the transplanted bone and the resection edges of the host bone in a 3-week autogenous bone graft. (b) Corresponding radiograph shows a not-yet-mineralized graft with a “cloudy” appearance indicative of a graft that is not yet consolidated. The radiolucent line between the graft and host bone is the result of a dying-back resorption of the host bone from periosteal reflection. (Reprinted with permission from Marx and Garg. 1)
Читать дальше