PDGFs, found in several types of human cells but mostly in platelets, were the first type of growth factor discovered in alpha granules. 27PDGF adheres only to target cells with receptive surface membranes. When a wound is treated with concentrated platelets, the release of PDGF triggers activity in fibroblasts, neutrophils, and macrophages, stimulating the latter to additionally release growth factors that help to heal injured tissues. 70–72Specifically, the target cells’ transmembrane receptors are activated by the platelet-released growth factors, and the receptors’ intracytoplasmic qualities activate the signal transducer proteins, one of which migrates to the target cell’s nucleus. There, the protein initiates a particular, regulated gene sequence—which may, for example, include the production of osteoid.
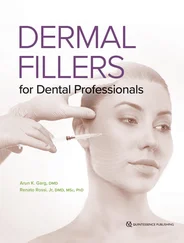
Alternatively, the sequence might lead to the synthesis of collagen. The regulated nature of the sequence precludes an “overdose” of concentrated growth factors. Growth factors’ amutagenic qualities testify to their ability as natural proteins to initiate and participate in the regular genetic activities associated with the controlled mechanisms for wound healing. 1,73–76As isomers of a single protein, and functioning as mitogens, PDGFs—the most common growth factors—perform different but often complementary tasks ( Fig 1-6). This prompts certain cells to replicate, specifically mesenchymal stem cells, osteoblasts (producing osteoid), endothelial cells (secreting basal lamina), and fibroblasts (producing collagen). 77–83

FIG 1-5Wound healing and tissue regeneration cycle.
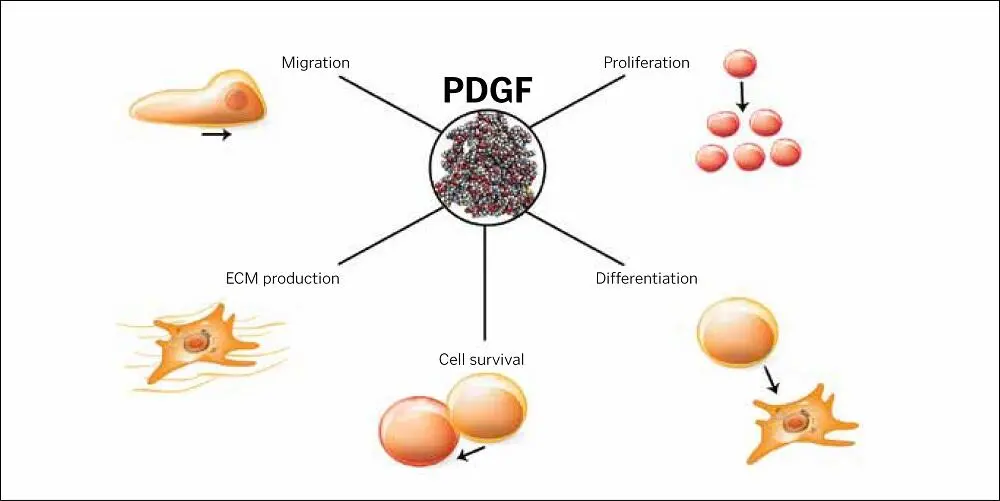
FIG 1-6Roles of PDGF.
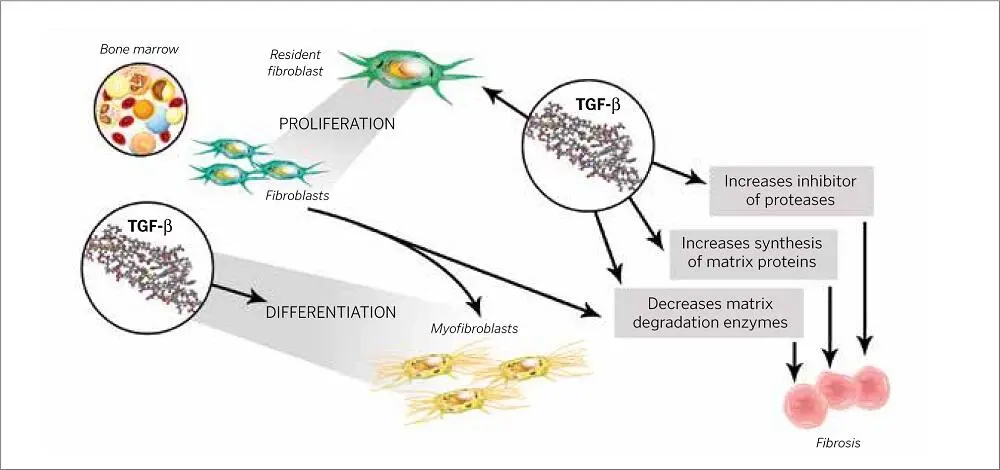
FIG 1-7TGF-β stimulates the proliferation and migration of fibroblasts and the process of epithelial-mesenchymal transition, but it also stimulates fibrotic responses, which can lead to end-stage organ failure in the heart, kidney, etc.
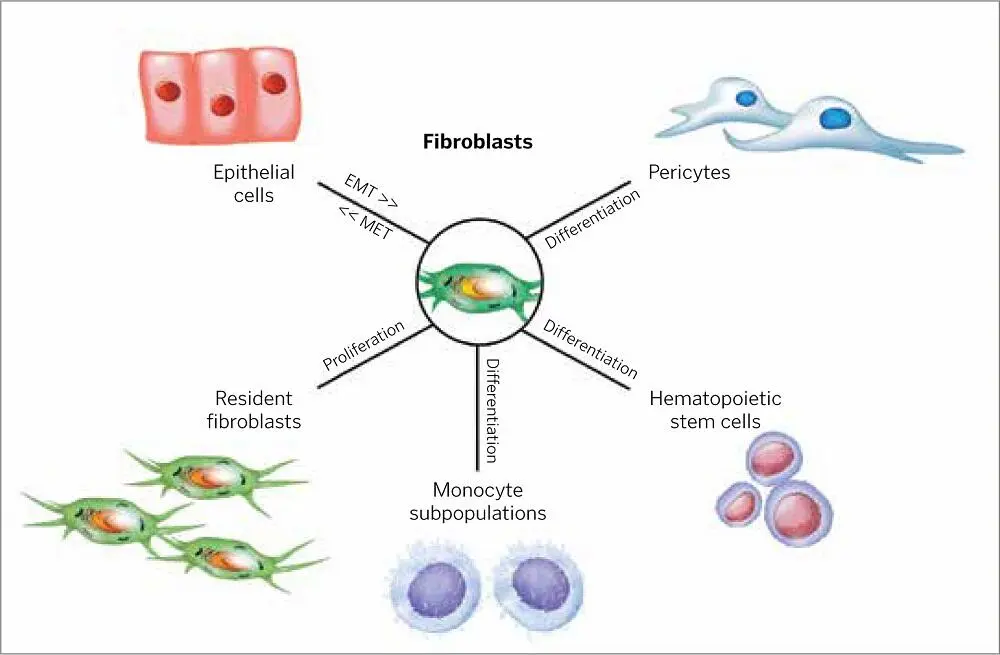
FIG 1-8Fibroblasts have different origins at different developmental stages (EMT, epithelial-mesenchymal transition; MET, mesenchymal-epithelial transition).
TGF-β1, TGF-β2, and TGF-β3
There are dozens of TGFs (including BMPs), and three of them (TGF-β1, TGF-β2, and TGF-β3) are protein growth factors that behave not only as mitogens (for cell replication) but also as morphogens (for cell differentiation). 84,85As cell preservers, TGF-β factors serve essential functions in wound healing, from the fetus to the adult. 86–89Like PDGFs, TGFs exert their influence in both healthy and pathologic cell activities, making their therapeutic use extremely problematic and challenging in that they often behave in opposite and even contradictory ways in both soft and hard tissues, including their roles in proliferation, migration, and differentiation 90–93( Fig 1-7).
FGF is an important mitogen inducer of fibroblast and endothelial cell proliferation. It also stimulates angiogenesis and plays a vital role in the repair of skeletal muscles and tendons. New blood vessel formation is due in large part to the activities of FGF, and it stimulates the migration of the macrophage as well as epithelium for epidermis formation 40,94,95( Fig 1-8).
IGFs are peptide hormones that have been found to promote cell growth in in vitro experiments. IGFs were also found to reduce levels of blood glucose in various tissues, hence their name. Their ability to stimulate glandular activities in humans differentiates them from other PRP growth factors. For example, IGF-1 mainly adheres to and stimulates receptors in cells of pituitary growth hormones. Most cells increase in size and number as a direct result of the synthesis and secretion of IGF-1 by tissues stimulated by growth hormones.
Growth factor production, along with growth hormone production and concentrations, wax and wane as part of the natural maturation process of humans before, during, and after pubescence, particularly for IGF-1. Liver production accounts for most IGF concentrations. IGFs have their most potent growth-stimulating effect on themselves and on nearby cells, and they (both IGF-1 and IGF-2) aid in bone cell mitosis. As an osteoblast secretion, IGF assists in osteogenesis and bone ossification via proliferation and differentiation of cells 96–98( Fig 1-9).
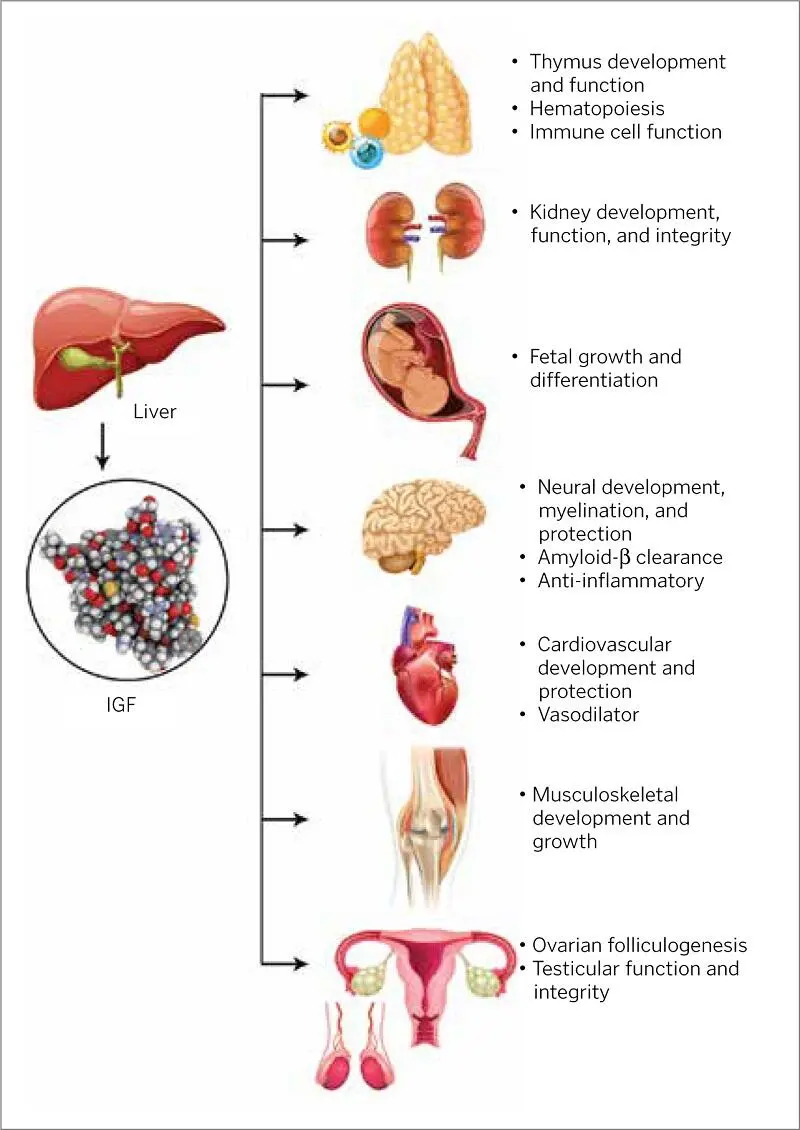
FIG 1-9Some of the roles of IGFs.

FIG 1-10VEGF stimulates the endothelial cell, enhancing multiple phases of the angiogenic cascade.
The protein-based EGF causes the basal cells of the skin and mucous membranes to replicate, migrate, and form essential elements of these membranes. 99EGF likely positively affects the generation of tissues as well as wound healing because of the way it controls the proliferation, growth, and migration of epithelial cells while strengthening the formation of new blood vessels. A therapeutic application of EGF has been used in preventing and treating dermatitis resulting from overexposure to x-rays or radium.
As its name suggests, the protein-based VEGF helps to develop blood vessels by interacting with endothelial cells, stimulating the synthesis of basal lamina, and recruiting pericytes 69,100( Fig 1-10). Like several other growth factors, VEGF is known for and studied scientifically mainly due to its activity in pathologic states rather than healthy ones. The role it plays in the formation of new blood vessels of cancerous tumors has provided much of what we know of its abilities to stimulate growth in other cells. Tumors, just like healthy cells, can synthesize growth factor proteins—including VEGF—that promote the formation of new blood vessels from existing ones (angiogenesis). Instead of angiogenesis taking place for normal body growth or tissue repair, in this pathologic process it enables the spread of cancerous cells. In this case, VEGF activity leads to the development of capillaries within the tumor because of its stimulation of endothelial cells, which are the raw materials needed for angiogenesis. Endothelial cell division leads to the growth and migration of the tumor cells, developing a cascade of shared growth factors between their cells. Possible cancer therapies, therefore, include ways to introduce proteins into the tumor that slow or stop angiogenesis.
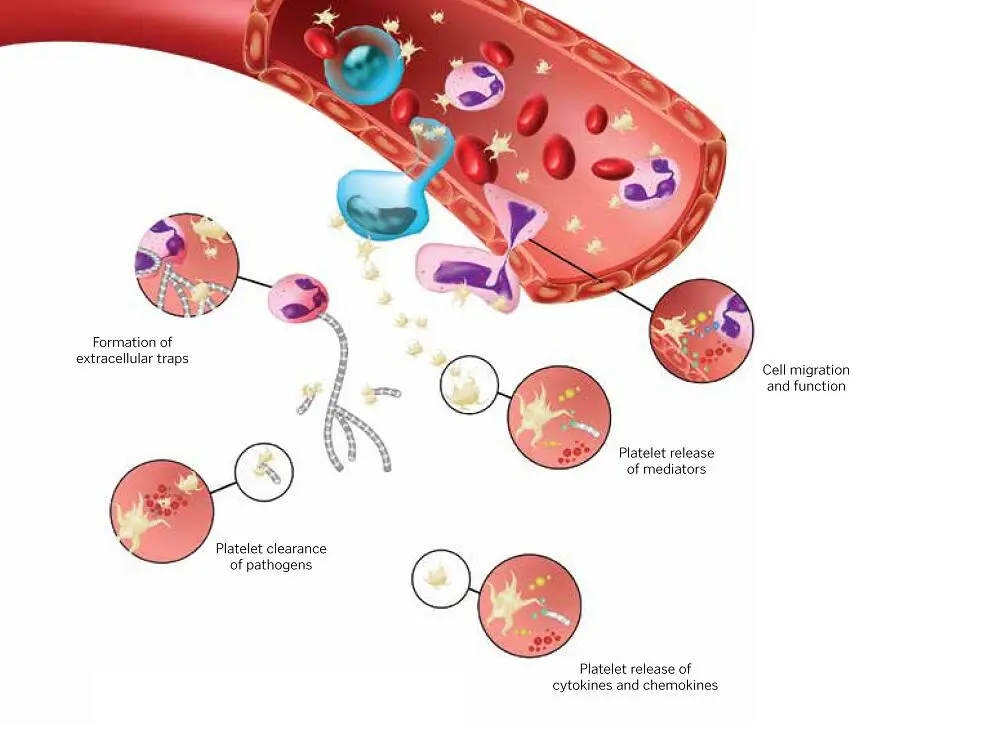
FIG 1-11Effects of platelet-leukocyte aggregates on differentiation, activation, and cytokine release.
Читать дальше