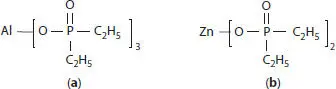A recent study compares the flame retardant efficiency of Ca-Hypo in polyamide 6, polylactic acid, thermoplastic polyurethanes and poly(methyl methacrylate) [156]. Interestingly, Al-Hypo was found to be efficient in polyolefins and styrenics. In these polymers phosphorus non intumescent flame retardants are typically inefficient. For example, Al- or Ca-Hypos combined with hindered N-alkoxyamines (NOR) stabilizers shows a V-2 rating in polyethylene, polypropylene and EVA [157]. More efficient are combinations of hypophosphite salts with brominated FRs, the most efficient of which seems to be melamine hydrobromide [158]. These synergistic blends allow achievement of a V-2 rating in PP copolymers at a level below 3 wt. % especially when combined with a free-radical initiator or NOR. At such a low loading, the content of bromine in the polymer is below 900 ppm, which qualifies it as halogen-free according to IEC 61249-2-21. Al-Hypo was found to be synergistic in combination with APP and a triazine based intumescent system in polypropylene [159] and with piperazine pyrophosphate in thermoplastic elastomers [160] and polyamide 6 [161]. Al-Hypo, combined with melamine cyanurate, liquid bisphosphate and about 2.5 wt. % phenolic novolac as a charring agent was found efficient in polyester thermoplastic elastomers [162]. 17.5 wt. % Al-Hypo, 7.5 wt. % resorcinol bis(2,6-xylenol phosphate) ( Formula 2.26) and 0.3 wt. % polytetrafluoroethylene (PTFE) as an antidrip agent provides a V-0 rating at 3.2 mm thickness in ABS [163]. APP, melamine cyanurate, cyclic phosphonate, magnesium hydroxide and even antimony trioxide were found synergistic with stabilized Ca-Hypo in achieving a V-0 rating in ABS[164]. Because Ca- and Al-Hypo provide gas phase flame retardant action, they can replace antimony trioxide in bromine-based systems in ABS [165] and glass-filled PBT [166].
The development of alkylphosphinate salts as flame retardants goes back to the late 70s and early 80s when various metal salts of dialkylphosphinates were tested in poly(ethylene terephthalate)[167] and in polyamide 6[168]. Later zinc, aluminum and calcium dialkylphosphinate salts were tested in glass filled polyamides and PBT. Aluminum and calcium ethylmethylphosphinates were found to give V-0 at 15 wt. % in plain PBT, at 20 wt. % in glass-filled PBT [169], and at 30 wt. % in glass-filled polyamides [170]. Because key raw material methyldichlorophosphine is strictly regulated, methylethylphosphinates were never commercialized but instead less expensive and safer to manufacture aluminum diethylphosphinate (DEPAL, Formula 2.4(a)) and zinc diethylphosphinate (DEPZN, Formula 2.4(b)) were developed [171].
(2.4) 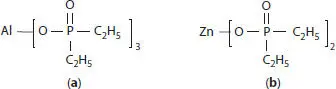
Although DEPAL is only moderately efficient in polyamides it was found to be synergistic with nitrogen-containing flame retardants such as melamine cyanurate [172], melamine phosphate or melamine polyphosphate[173]. Further addition of a few percent of zinc borate as a stabilizer is required for high temperature processing of polyamide 6.6 [174]. These products provide UL-94 V-0 ratings in glass-filled polyamides at 15-20 wt. % loading down to 0.4 mm thickness [175]. Melamine polyphosphate with some melamine replaced by Mg, Zn or Al were also tested as more thermally stable synergists to DEPAL [176]. Another synergistic combination of DEPAL with aluminum phosphite (AlPO 3, PHOPAL) was found to be highly efficient in polyamides [177, 178] and it is believed to be used commercially. More thermally stable boehmite (AlOOH) in combination with zinc borate was recommended for high melting semiaromatic polyamides [179] and also shows synergism in PBT [180]. Surprisingly, high aspect ratio talc was also found to be synergistic with DEPAL in polyamide 6 [181]. Since DEPAL is efficient in polyamides it was also found to be efficient in polyphenylene ether (PPE) polyamide blends [182].
It seems there is no actual synergism between DEPAL and melamine salts in glass-filled PBT, but about 1/3 of DEPAL can be replaced with melamine cyanurate or melamine polyphosphate without loss of the V-0 rating [175], which is probably beneficial for cost saving. Compared to brominated flame retardants used in the same application, DEPAL allows a high Comparative Tracking Index (CTI) > 500 volt [183, 184]. On the other hand, DEPAL and DEPAL-based synergistic combinations show significant wear (corrosion) of processing equipment [185], which can be decreased by using acid scavengers.
Based on academic studies there is a strong indication that DEPAL mostly operates in the gas phase by a flame inhibiting mechanism [186, 187]. It was believed that DEPAL decomposes with the evolution of phosphinic acid which evaporates to the flame, however there is other evidence showing that aluminum alkylphosphinates can volatilize without decomposition. Interestingly, under thermooxidative conditions of thermogravimetric analysis in air DEPAL doesn’t volatilize, but mostly oxidizes to aluminum phosphates [188]. The higher volatility of the salt results in a higher flame retardant efficiency as demonstrated by aluminum diisobutylphosphinate [189, 190]. Melamine polyphosphate provides a condensed phase action by increasing the charring of the polymer [191] and thus provides a synergistic action with DEPAL [186]. In contrast, melamine cyanurate mostly volatilizes and provides a cooling effect to the flame [187], therefore its action is mostly adjunctive but not synergistic. A comparative study of DEPAL and Al-Hypo plus resorcinol bis(2,6-xylenol phosphate) ( Formula 2.6) showed that DEPAL is more efficient at 20 wt. % loading in glass filled PBT [192].
Apart from traditional uses of DEPAL in polyamides and polyesters it was also found alone [193] or in combination with melamine polyphosphate [194] or with aromatic bisphosphates [195] to be efficient in PPE/styrene-ethylene-butadiene-styrene (SEBS) blends typically used in cable jackets. About 30 wt. % DEPAL is required to achieve a VW-1 rating in thermoplastic elastomers (TPE) wire insulation [196] and the same rating can be achieved if about 1/3 of the DEPAL is replaced with melamine polyphosphate [197] or melamine cyanurate [198]. Similarly, in thermoplastic polyurethane (TPU) about 30 wt. % DEPAL and some melamine salt allow achievement of a VW-1 rating [199].
Apparently, DEPAL alone [200] or in combination with ATH, APP or melamine [201] or melamine polyphosphate[202] shows high efficiency in unsaturated polyesters (UPE). For example, a combination of 10 wt. % aluminum diethylphosphinate and 10 wt. % melamine polyphosphate provides a V-0 rating in a 30% glass-filled composite and shows an LOI of 42. DEPAL can also be pre-dispersed in a polyester/styrene prepolymer [203] which results in higher LOI values compared to freshly added DEPAL. Because DEPAL doesn’t dissolve in epoxy resin and behaves as a flame-retardant filler, its finely milled grade is useful in low loss factor compositions [204]. Pre-dispersion of DEPAL in epoxy [205] or use of multifunctional highly charrable epoxy resin [206] or combinations with melamine polyphosphate [207] help to boost the efficiency of DEPAL. Because non epoxy based printed wiring boards have an even lower loss factor, DEPAL became a popular flame retardant in compositions based on polyphenylene ether[208] or bismaleimide [209].
In contrast to DEPAL which melts with decomposition above 400°C, zinc diethylphosphinate (DEPZN) melts at about 220°C and therefore it was claimed [210] to be particularly suitable for fiber and film additive applications, for example to decrease the heat release rate of PET textile [211]. A recent patent [212] suggests that DEPZN is useful in poly(trimethylene terephthalate) fibers for carpeting. It can also be melt processed with semi-aromatic polyamide which is blended with rayon fibers to pass the ASTM D6413 test [213] or with TPU to produce wrapping tape passing the automotive FMVSS 302 test [214]. A recent academic publication [215] shows that 8 wt. % DEPZN can decrease the ATH loading from 60 wt. % to 37 wt. % in UPE in order to achieve a V-0 rating.
Читать дальше