Corrosion Policy Decision Making
Здесь есть возможность читать онлайн «Corrosion Policy Decision Making» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Corrosion Policy Decision Making
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Corrosion Policy Decision Making: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Corrosion Policy Decision Making»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the science, management, economy, ecology, and engineering of corrosion management and prevention Corrosion Policy Decision Making
Corrosion Policy Decision Making
Corrosion Policy Decision Making — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Corrosion Policy Decision Making», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
All that calls for being constantly alert to consider the option for a design change‐modification. In this context, design modification means that the existing design is altered with minimum costs, whereas change may mean complete make‐over, which would of course bring many costs.
The policy is that before opting for design change‐modification/materials selection, do a thorough feasibility study to compare this option with the economy and possible outcome with the other four options (when applicable), and then decide if you want to continue with it or leave it, either temporarily or permanently.
Yet another strategy in this respect could be increasing the thickness of the metal. This technique is technically referred to as creating “Corrosion Allowance.” As the very name implies, the method is essentially based on increasing the thickness of the metallic asset. This way we actually “allow” corrosion to work its way through a certain thickness of the asset before too much corrosion of the thickness occurs. It is important to note that corrosion is essentially effective on the thickness, as it is this dimension that on a larger scale carries the stress imposed on the material. Corrosion allowance is applied not to treat corrosion but to delay it. In addition, this method can be taken as a practically useful measure should the form of corrosion be uniform. However, as it may occur with many corrosion process types, localized corrosion in the formation of pits leads to deepening of these pits and results in through‐wall leak, and failure of coalescence of the pits leads to cracks and thus lowers the threshold of tolerating applied stress that can occur. Corrosion allowance is not widely used except in occasions such as submarine (deep and ultra‐deep) pipelines and pressure vessels. 3 On such occasions, the other four corrosion treatment strategies may not applicable all the time (such as installing cathodic protection [CP]). However limited the number of industries that use corrosion allowance, it is still an especially important option for a better corrosion treatment approach.
Materials selection is a very expensive option to control or prevent corrosion (see Chapter 3to understand the difference between corrosion control and corrosion prevention). In this option, the existing material of an asset is replaced with a more corrosion‐resistant material so that the overall service life of the asset is increased. Figure 2.5shows an example of materials selection for upstream oil and gas industry:
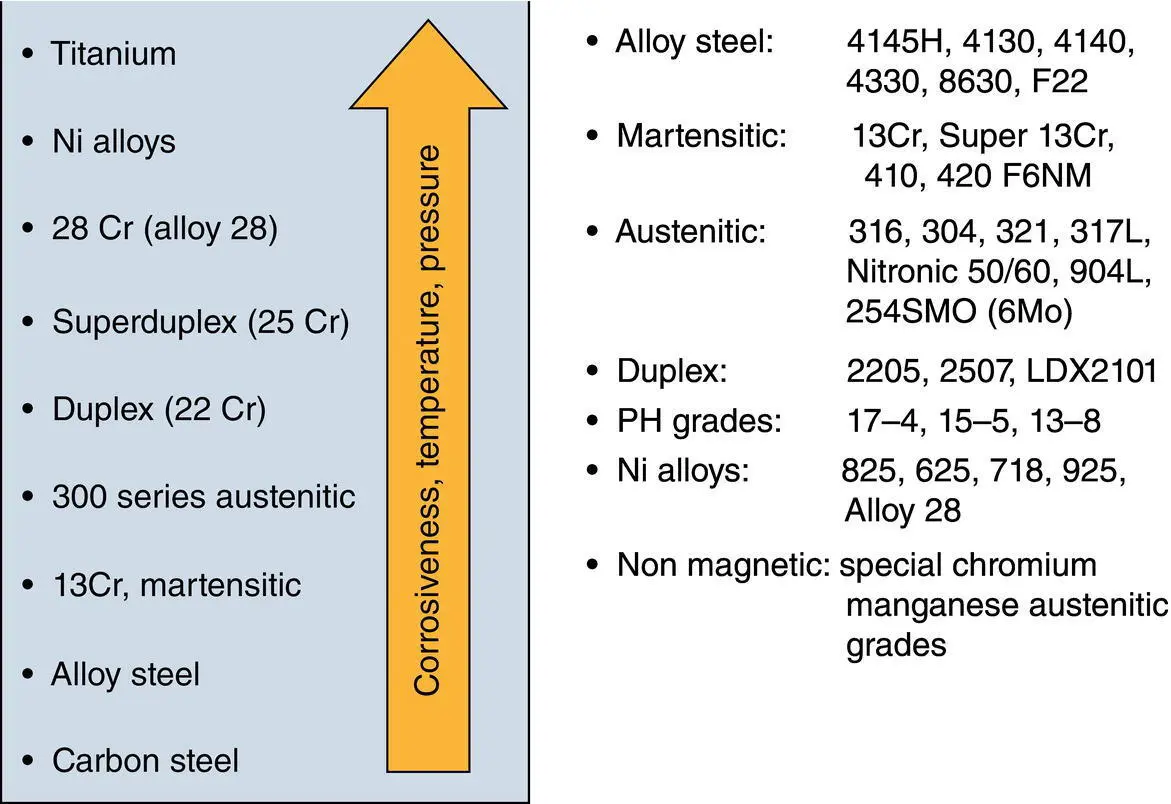
Figure 2.5 Materials selection chart for upstream exploration oil and gas industry.
While materials selection sounds like a viable option in treating corrosion by replacing less corrosion resistant materials with more resistant ones, one incredibly significant point to remember is its feasibility. In other words, while project managers may accept the superiority of the selected material over the existing material from a corrosion treatment strategy point of view, an important drawback could be the extra cost imposed by this option to the overall cost of the project. One has to bear in mind that the cost is not limited to the cost of the selected material per se, it is the person‐hours needed to replace and operate, and the downtime between these steps. Say that we have decided to replace a carbon steel part with a stainless steel part. The price difference for January 2021 has been shown in Figure 2.6, where the overall difference is about 40% per mass of the steel selected. This can be translated to huge extra costs for shifting from carbon steel to stainless steel.
The issue will even become more considerable for projects that are being carried out in countries whose currency is not US dollars. When translated into their national currency, the imposed cost may become more eye catching, rendering it more likely to get negative responses to finance a materials selection option. Therefore, it is of vital importance to convince the management not only in terms of corrosion treatment‐related terms but also in terms of the service life extension via an economic analysis such as life cycle cost analysis (we have briefly explained this and other models in Chapter 3[Smart Corrosion Management Elements] and in more details related to economy in Chapter 4[A Short Review of Some Fundamental Principles of Economics]).
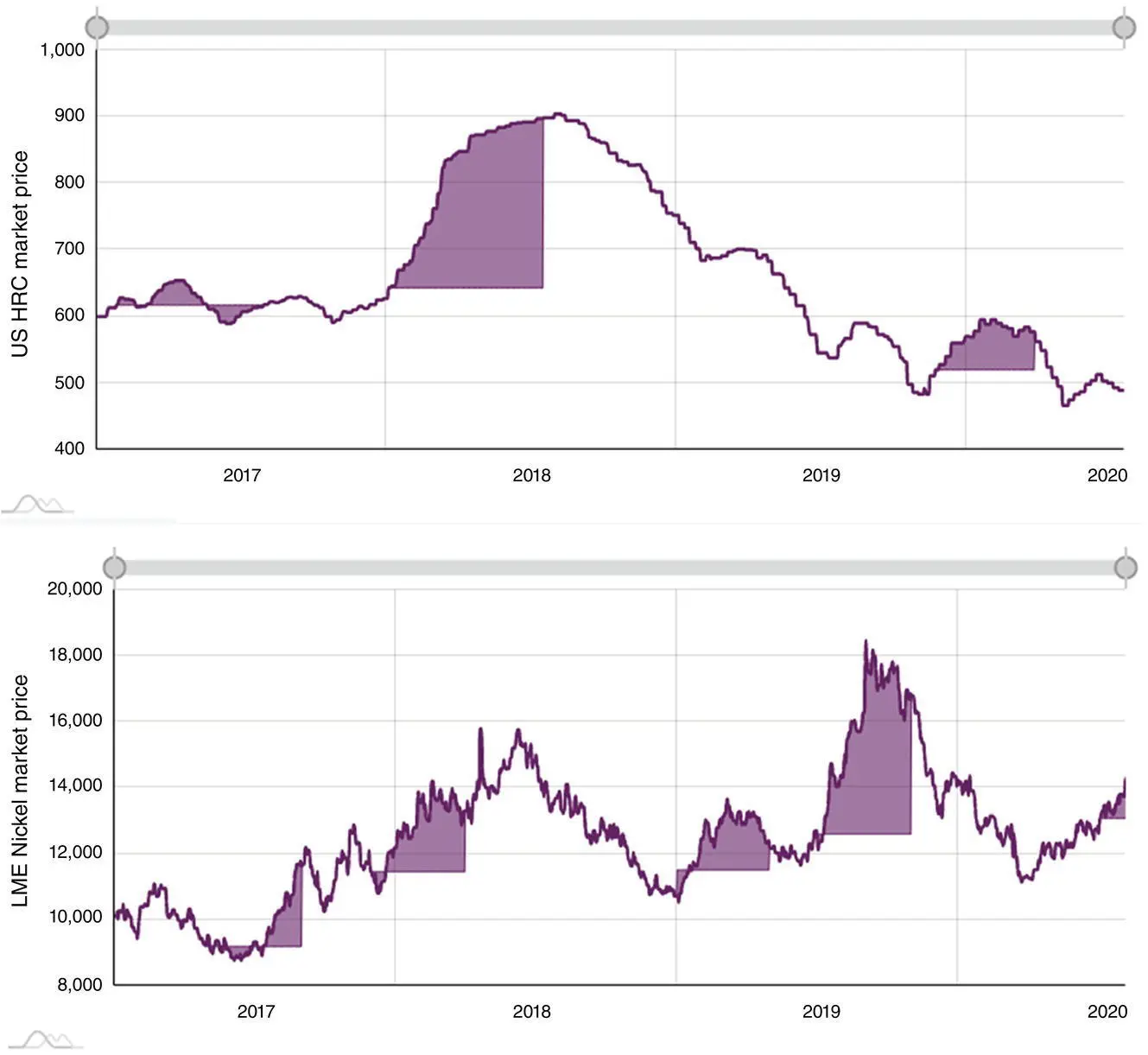
Figure 2.6 Carbon steel (hot rolled, cold rolled, and galvanized coils) price fluctuations with an average price of $0.5027/Ib (top). Stainless steel (sheet) price fluctuations with an average price of $1.219/Ib (bottom) [1] (all average prices based on prices given on 1 January 2021).
Change or modification of design can be performed without a need for materials selection if applied smartly. For instance, where possibility of galvanic corrosion exists, it is possible that by either changing the anode/cathode ratio, applying CP (to reverse the polarities), or coating either anode or cathode (or sometimes both) solve the corrosion treatment issue as a corrosion prevention/control approach.
Last but not least, the option for design modification‐change/materials selection could be an option which is paradoxically both inexpensive and expensive; cost of design per se may not become too unbearable, whereas costs imposed by materials selection can become so exceedingly high that the project management will be pushed to consider other measures. Therefore, in dealing with corrosion treatment via this option, any proposal for doing so should come with a smartly planned economic analysis for whole process and not just relying on decreasing corrosion rates and severity alone.
2.2.2 Chemical Treatment
One the ways by which corrosion can be brought under control is application of corrosion inhibitors, and in case of MIC, biocides. The reason we emphasize mentioning biocides along with corrosion inhibitors is the incorrect belief by some corrosionists that by applying inhibitors, MIC can also be treated, or rather, MIC is less important than non‐MIC corrosion problems. While MIC is also an electrochemical corrosion in essence, corrosion inhibitors (or nearly all of them) cannot treat MIC, as the contributing factor to corrosion is micro‐organisms.
Corrosion inhibitors can be grouped by selecting various criteria, but we prefer to categorize them into main three groups; anodic, cathodic, and mixed effect inhibitors.
Anodic inhibitors form a protective oxide film on the surface of the metal. This film will be instrumental in causing a large anodic shift of the corrosion potential. This shift forces the metallic surface into the passivation region where the material can be assumed to become immune to corrosion. Due to this effect, anodic corrosion inhibitors are also sometimes referred to as passivators. Chromates, nitrates, tungstate, and molybdates are some examples of anodic inhibitors. On the other hand, cathodic inhibitors act mainly via two mechanisms; either by slowing the cathodic reaction itself, or selectively precipitating on cathodic areas to limit the diffusion of reducing species to the surface. The mechanism of mixed effect corrosion inhibitors can be summarized as the three points below:
Mixed inhibitors work by reducing both the cathodic and anodic reactions. They are typically film‐forming compounds that cause the formation of precipitates on the surface, blocking both anodic and cathodic sites indirectly.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Corrosion Policy Decision Making»
Представляем Вашему вниманию похожие книги на «Corrosion Policy Decision Making» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Corrosion Policy Decision Making» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












