Corrosion Policy Decision Making
Здесь есть возможность читать онлайн «Corrosion Policy Decision Making» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Corrosion Policy Decision Making
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Corrosion Policy Decision Making: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Corrosion Policy Decision Making»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the science, management, economy, ecology, and engineering of corrosion management and prevention Corrosion Policy Decision Making
Corrosion Policy Decision Making
Corrosion Policy Decision Making — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Corrosion Policy Decision Making», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
It is also evident that we still have the restrictions imposed by the specific temperature that must be maintained in ordering anodic and cathodic reactions for the given environment. Furthermore, what is to be noticed with regards to the galvanic series given in Figure 2.3the same as electrochemical series ranking, any reaction which is placed above another reaction is regarded cathodic to that reaction. For instance, while in Figure 2.2, aluminum reaction was more cathodic with respects to magnesium, in Figure 2.3, bronzes are regarded more anodic with regards to copper–nickel (70–30). It follows that while electrochemical and galvanic series may differ in many respects, it is still the ranking of a particular reaction with regards to the other one that determines if it is noble (cathode) or active (anode).
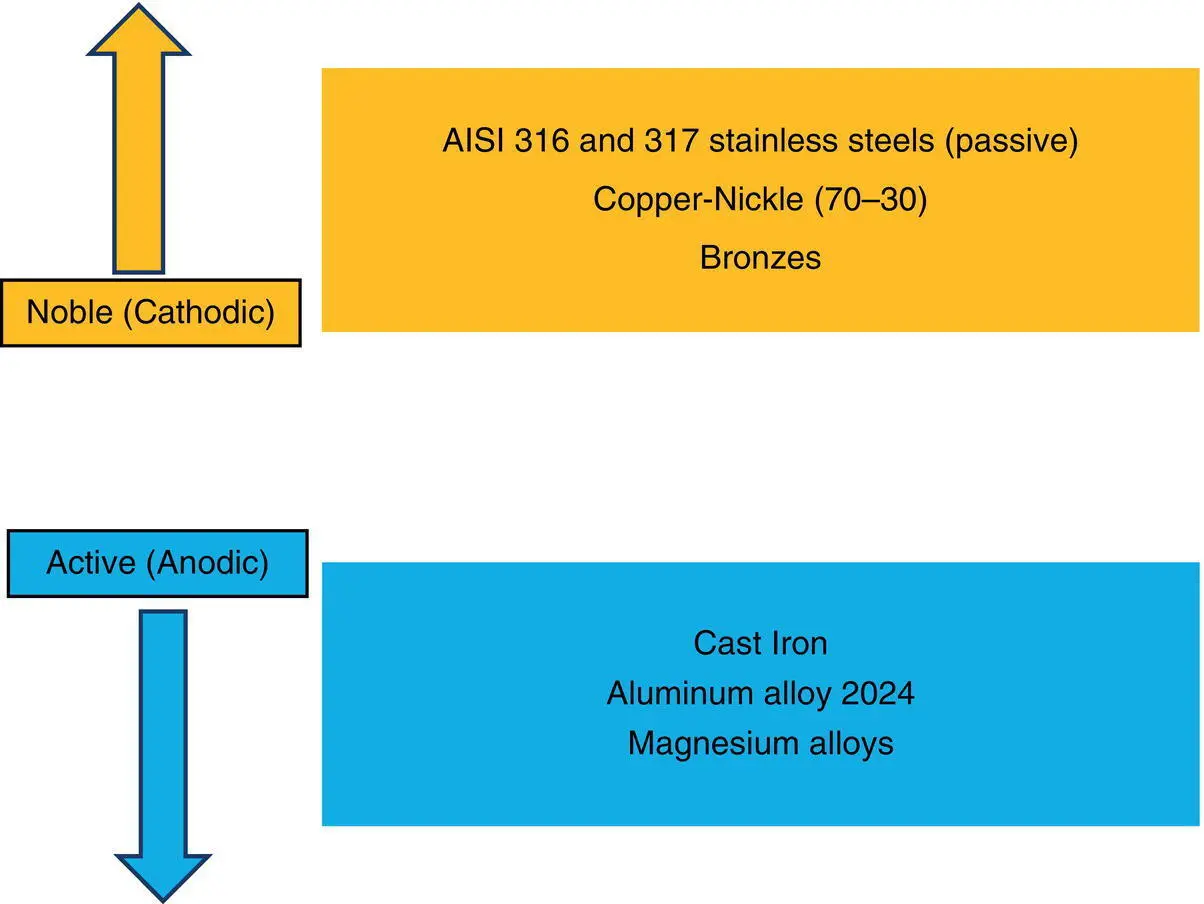
Figure 2.3 Some examples of active and passive metals in seawater at 25 °C for the specific environment seawater.
As we mentioned earlier, although galvanic series seem to be more practical than electrochemical series, there are still some limitations. Being environment‐ and temperature‐specific is an inescapable limitation on all chemical reactions as they do happen under certain conditions, the most important of which are the electrolyte (that is, the environment), the temperature (as chemical reactions are kinetics highly dependent on temperature), and of course, pressure. However, there is yet another measure that can assist us in predicting corrosion behavior of metallic alloys and it was invented by a PhD student some decades ago, named Marcel Pourbaix. 2
2.1.2.3 Pourbaix Diagrams
An example of a Pourbaix diagram is shown in Figure 2.4.
Pourbaix diagrams have two axes, one for corrosion potential as measured in hydrogen potential, and one for measuring acidity of the environment shown as pH. For a given set of potential‐pH‐environment, some “domains” will be created. These domains can be used to predict, under the given conditions for the three parameters mentioned above, if it is possible to expect corrosion or immunity to corrosion (in other words, passivity). Existence of some corrosive ions such as, but not limited to, chlorides, can somehow change the domains and thus shift the potentials in which corrosion or passivation can be expected.
One very important aspect of Pourbaix diagrams in addition to enabling us to predict safe and unsafe values of potential and pH with regards to corrosion for a given environment is to put emphasis on what is almost forgotten by a majority of non‐corrosion expert professionals; it is normally assumed that as long as we know about the pH of the environment, it is safe to say if it is corrosive or not! The rule of thumb for these self‐acclaimed corrosionists is that if pH is below 7 the environment is acidic and thus corrosive, if pH is 7 it is neutral, and if it is larger than 7 the environment is basic. This is wrong! In order to interpret correctly, one has to know both corrosion potential and pH. As an example, take the Pourbaix diagram in Figure 2.4when chloride is present; at an acidic pH = 6 and potential = −0.6 V (red dashed line intersection in Figure 2.4), there is immunity against corrosion, whereas in the same system, but this time for a neutral pH = 7 and potential = −0.4 V, corrosion is highly likely to happen (black dashed line intersection in Figure 2.4). This alone can serve to show how powerful Pourbaix diagrams are in dealing with corrosion and predicting it. However, as noticed by our readers, the restrictions due to temperature do still remain.
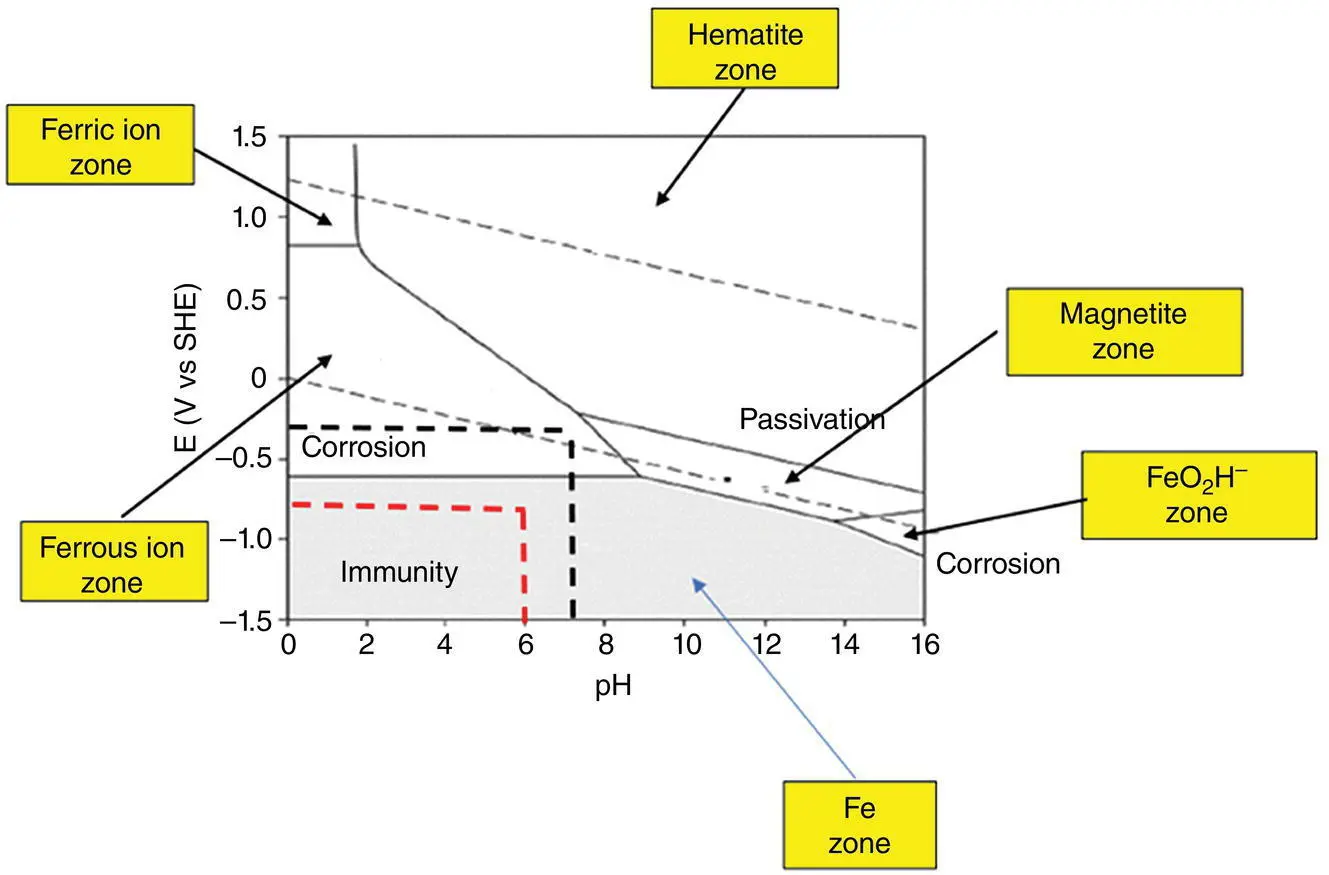
Figure 2.4 A typical Pourbaix diagram (simplified) for an Fe–water system at iron concentration of 10–6 mol/l and 25 °C. The region between the two dashed lines is the water stability zone. The two dashed lines, upper and lower, define the domains for oxygen and hydrogen stability; above the upper line water is oxidized to O 2, so oxygen is evolved above the upper line. Below the lower line, water decomposes to H 2and thus, hydrogen will be liberated below the hydrogen line. The zones shown indicate the chemical species formed. Red and black dashed lines, show immunity under acidic and corrosion under neutral pH values, respectively, emphasizing the necessity for specifying and studying pH–voltage pair to decide about possible corrosivity scenarios. Hematite: Fe 2O 3; Magnetite: Fe 3O 4; Ferric ion: Fe +3; Ferrous ion: Fe +2.
2.2 Important Technical Treatment Strategies for Corrosion Treatment
Corrosion treatment strategies can be categorized into five categories:
1 Design modification‐change/materials selection (change and/or modification of design including change/upgrading of materials).
2 Chemical treatment; use of various kinds of corrosion inhibitors (anodic inhibitors, cathodic inhibitors and mixed effect inhibitors, biocides either as oxidizing biocides or non‐oxidizing biocides).
3 Electrical treatment (the most noticeable example in this context is cathodic protection).
4 Mechanical treatment (pigging).
5 Physical treatment (paints/coatings).
As we can see, the corrosion treatment strategies and techniques are indeed very limited in number. However, depending on the corrosion process in focus, there can exist some alterations. For example, if the corrosion case happens to be MIC, then yet another option can be added; the biological treatment of MIC. These alterations do not mean that the essential five strategies cannot be applied. For instance, in the case of MIC, physical treatment as well as electrical and mechanical treatment may also be applicable. The alterations are only applicable where, due to the very nature of the corrosion process, more treatment options may be made available.
Below we will briefly review these five treatment strategies. Once again, we should remind our readers that neither this chapter nor the book itself is about teaching corrosion science and the electrochemistry behind it in the way that many famous handbooks and textbooks do. Our main focus here is on knowing as much necessary for an engineer who happens to become responsible for developing a CM strategy and who may need to know some basic, important elements of the science of corrosion when trying to differentiate between CM various models. As we have described in full in Chapter 3under the title of “Smart Corrosion Management Elements,” having an efficient, realistic, smart model for CM is an integral part of any strategy that seriously considers management of corrosion.
2.2.1 Design Modification‐change/Materials Selection
Contrary to what many engineers and operators may think, the as‐is design of assets is not the last word when it comes to CM of the asset. In fact, many factors during fabrication, welding, coating, testing, and operation may become the points at which corrosion can be invited. For instance, it is usually recommended to use continuous welding instead of spot welding to avoid problems such as moisture entrance, or having as few branches as possible in a pipeline to avoid stagnation points. In addition, it is highly likely that MIC problems can occur in post‐hydrostatic testing of pipelines, which is mainly applied to test the welding strength (contrary to pneumatic test which is essentially a leak test, hydrotest is both a strength and leak test).
Yet another issue that may arise is to have formed galvanic cells by joining two metals that each can have different electrochemical properties (such as welding together the same metals with and without coating, or coupling dissimilar metals to each other so that in addition to creating a potential difference, anode/cathode area ratios less than one will be created). Operation conditions may lead into initiation and development of MIC in the asset, an example can be seen for example, in underground firewater rings or within reverse osmosis membranes systems. Examples are numerous.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Corrosion Policy Decision Making»
Представляем Вашему вниманию похожие книги на «Corrosion Policy Decision Making» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Corrosion Policy Decision Making» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












