Corrosion Policy Decision Making
Здесь есть возможность читать онлайн «Corrosion Policy Decision Making» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Corrosion Policy Decision Making
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Corrosion Policy Decision Making: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Corrosion Policy Decision Making»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Explore the science, management, economy, ecology, and engineering of corrosion management and prevention Corrosion Policy Decision Making
Corrosion Policy Decision Making
Corrosion Policy Decision Making — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Corrosion Policy Decision Making», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
It is quite understandable if these very concepts that form electrochemical corrosion—or more generally referred to as aqueous corrosion—leave a lot of questions for the readers. For example, from a spatial point of view, while the position of anode is visible how can we decide where exactly the cathode adjacent to it is? Is it on the left side of the anode? Its right side? Where is it located? Another matter is how do we know if the anode and cathode “creep” relative to each other on a metallic surface? And, for that matter, how do we know if one particular spot and not another spot on the metal will become the anode?
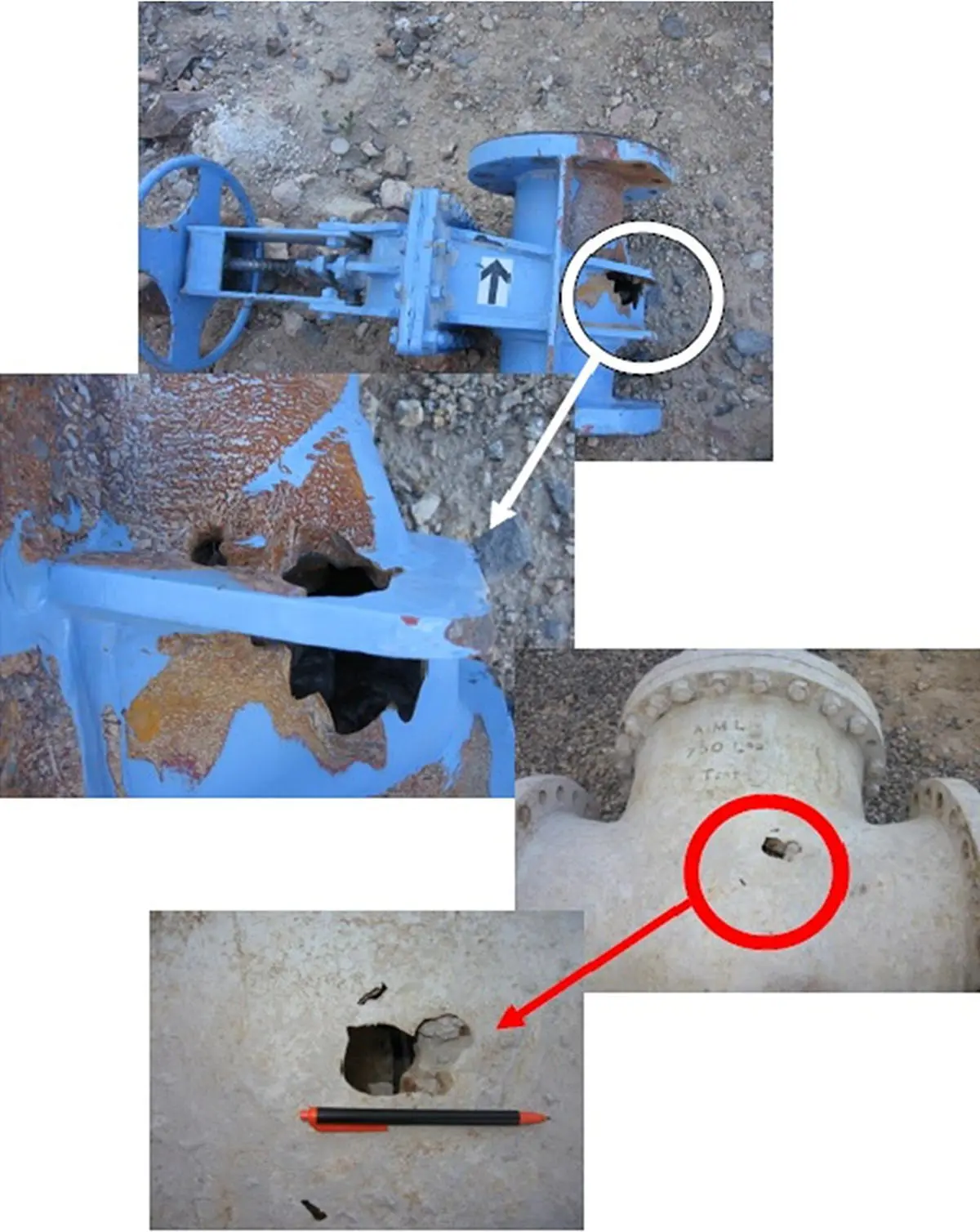
Figure 2.1 Two examples of severely corroded equipment leading into a through‐wall localized failure. The anode is the point at which perforation has occurred (white arrow) and the cathode is immediately adjacent to it so that electron flow happens via the metallic surface (orange arrow), while ionic transfer happens through the moisture layer already formed on the metal.
( Source: Taken from the collection of Dr. R. Javaherdashti.)
The above questions are actually some of fundamental importance should we be writing this book as a textbook. There are numerous books and papers that may become handy in answering these questions and even more puzzling ones. For us, however, the above questions carry no value at all. From a practical point of view, all we need to care about are the following five basic concepts:
1 At anode, anodic reactions happen. These reactions involve emitting the extra electron given to the metal through extractive metallurgy processes.
2 These electrons will be taken by the cathode. In other words, electrons emitted by the anode will be gained at the cathode, leading to the establishment of an electron flow (electricity) between anode and cathode.
3 The electron flow constitutes what we refer to as “current.” The current thus produced is in fact the way by which voltage difference can be leveled between anode and cathode.
4 The moisture already established on the metal's surface acts as a carrier medium for the exchange of ions thus produced as a result of anodic and cathodic reactions. In other words, the electron emittance and electron gaining will serve to produce anion and cation ions which can be carried through the moisture layer (the electrolyte).
5 It follows, then, contrary to what textbooks on corrosion try to impose on our understanding saying that there need to be four elements (the anode, the cathode, the electrolyte, and the metallic path) for a electrochemical corrosion scenario to exist, there are actually only three elements necessary; these are the anode, the cathode, and the electrolyte. As corrosion is already happening on a metallic asset, we really do not require to make more confusion that that is already existing in the electrochemistry of corrosion. As some may already know, we may name a few of these confusions; one can name the concept of using the wrong term of oxidation instead of anodic reaction, the polarity signs of anode and cathode, the arguable term of under deposit corrosion, and even the term biofilm to explain the venue at which electrochemical cells are established. 1
The above fundamental concepts explain both the science required to explain how corrosion occurs as well as the science needed to explain its prediction and treatment, as we will see in the next sections.
2.1.2 Prediction of Corrosion
We obviously need to know when corrosion happens, as this will enable us to prepare ourselves with the upcoming event and try to manage it as much as possible, in the best way achievable. As we have mentioned earlier, this is not a textbook about corrosion, and therefore many of the concepts that would have been covered in length in those books will be treated in the most practical approach possible to make these concepts as understandable for a corrosion specialist as possible.
There are mainly three tools that can be used to help us predict corrosion, in other words, to let us determine anodic and cathodic reactions and therefore find out about the possibility of corrosion.
These tools are:
1 standard hydrogen electrode scale (SHE)
2 galvanic series
3 Roubaix diagrams
By using these tools, it is possible to find out what chemical reactions can be considered as anodic and therefore giving off their extra electrons, and which reactions can be cathodic reactions to receive and gain those electrons. Obviously, the substrate on which these reactions will take place is a metallic surface (to allow electron transfer), and the environment will be water or water containing (the necessary electrolyte for ionic transfer).
2.1.2.1 Standard Hydrogen Electrode/Electrochemical Series
The main assumptions that need to be made are as follows.
1 It is assumed that the reduction voltage for hydrogen reaction is zero. In other words, it is assumed that the conversion of hydrogen ion (H+) to hydrogen gas is zero. Needless to say that the actual voltage is not zero at all, but this assumption is accepted so that some chemical reactions can be placed above hydrogen evolution (due to their positive reduction voltage), and some reactions will be placed below that due to their negative potential. Those with positive potential will be noble, meaning that they are very highly likely to act as a cathode and those with negative potentials will be branded as active, meaning that they are very highly likely to act as an anode.
2 All of the substances taking part in the electrode reaction have unit activity.
3 Temperature is 25 °C.
4 Hydrogen pressure in the reference electrode is one atmosphere. Figure 2.2 Schematic presentation of electrochemical series with some reactions as cathodic and anodic reactions. Standard potentials (Eo) are in volts vs. SHE (standard hydrogen electrode).
Figure 2.2schematically shows an example of an electrochemical series:
However, it is evident that items ii–iv can only be achieved under strict laboratory conditions and under real life, industrial conditions it is not possible to main temperatures and pressures as required by the electrochemical series. It can be said that it is mainly due to these restrictions as dictated by laboratory‐controlled conditions that industrial application of electrochemical series must be replaced with a more application‐friendly option. Although, as we see later, standard hydrogen potential is a necessary element in constructing Pourbaix diagrams, which are very useful in applications.
2.1.2.2 Galvanic Series
Due to limitations of hydrogen electrode measurement for constructing electrochemical series, another option has been applied which is called “electrochemical series.” The potentials used in a galvanic series are only valid in:
1 a given environment (electrolyte), and
2 at a given temperature (25 °C).
Temperatures can be corrected for any given environment, but the importance is to know what environment we are talking about. An example of a galvanic series for seawater at 25 °C can be seen in Figure 2.3.
As it can be seen from the figure, some reactions are still considered as cathodic and anodic; mainly those sitting near to the top of the galvanic series are considered noble (cathodic) and those ranked below are considered active (anodic). While the range of reactions for constructing a galvanic series for seawater at 25 °C is not limited to the few examples given in Figure 2.3, it is obvious that due to environment–specific property of galvanic series, these reactions hold true only for the electrolyte, that is to say, the environment for which they have been constructed.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Corrosion Policy Decision Making»
Представляем Вашему вниманию похожие книги на «Corrosion Policy Decision Making» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Corrosion Policy Decision Making» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












