The history and clinical examination should enable you to formulate a list of differential diagnoses. It may help to create a problem list, identifying the relevant historical features and predominant clinical signs, categorising them as contagious or non‐contagious, and allocating the disease within the following groups, which form the basis for the problem‐orientated approach in this book:
Pruritic
Crusting and scaling
Ulcerative and erosive
Nodular or swollen
Alopecia/hair coat changes
Pigmentary disorders
A diagnostic plan can then be constructed, diagnostic procedures selected, and samples collected. Sample collection may include the following techniques.
Useful to determine whether the lesions of alopecia or hypotrichosis are due to self‐inflicted damage (fractured hair shafts, split ends) indicating that the condition is pruritic, or due to abnormal hair growth (absence of anagen roots, abnormal catagen roots), and to examine for dermatophytes and for parasite eggs.
Choose fresh, unmedicated lesions.
For suspected dermatophytosis, where cultures are required, first lightly clean the areas to be sampled with 70% alcohol (to reduce contaminant organisms).
Tissue or epilation forceps can be used to grasp gently and pull out hairs from the periphery of the lesion.
Samples for microscopy can be placed on adhesive tape wrapped around a microscope slide and mounted in a drop of liquid paraffin just prior to examination.
Samples for fungal culture submission should be held in paper or sterile, non‐airtight containers to prevent a humid environment that might support the growth of saprophytic organisms.
Useful for cytological examination looking for bacterial organisms (particularly Dermatophilus ) and for submission for fungal and bacterial culture.
Choose a fresh, unmedicated lesion.
Impression smears of the underside of freshly removed crusts, stained with a rapid Romanowsky‐type stain (e.g. Diff‐Quik, Hemacolor, Rapi‐Diff, Speedy‐Diff) or Gram’s stain, can provide a quick method of diagnosis for dermatophilosis.
Crusts can be collected and held in paper envelopes or sterile containers for transport to the laboratory.
Dried crusts can be emulsified in a drop of sterile saline on a slide, warmed to allow rehydration of material, prior to air‐drying and fixing (heat fixation for Gram’s stain, methanol/ethanol fixation for rapid‐differentiating Romanowsky‐type stains) for cytological examination and identification of bacterial and fungal organisms.
These allow for examination for surface‐living external parasites and dermatophytes where the lesions are diffuse or extensive. Scrapings are better for deeper resident mite infestations.
Use a sterile scalp brush or new toothbrush to brush firmly over the lesions (Mackenzie brush technique; Figures 1.4a and b). Place the brush in a paper envelope to protect it prior to submission for dermatophyte culture.
A scalp brush or wooden tongue depressor can be used to collect debris directly into a sterile Petri dish for external parasites. Material should be examined promptly as chorioptic mange mites are highly motile and easily lost from sample containers.
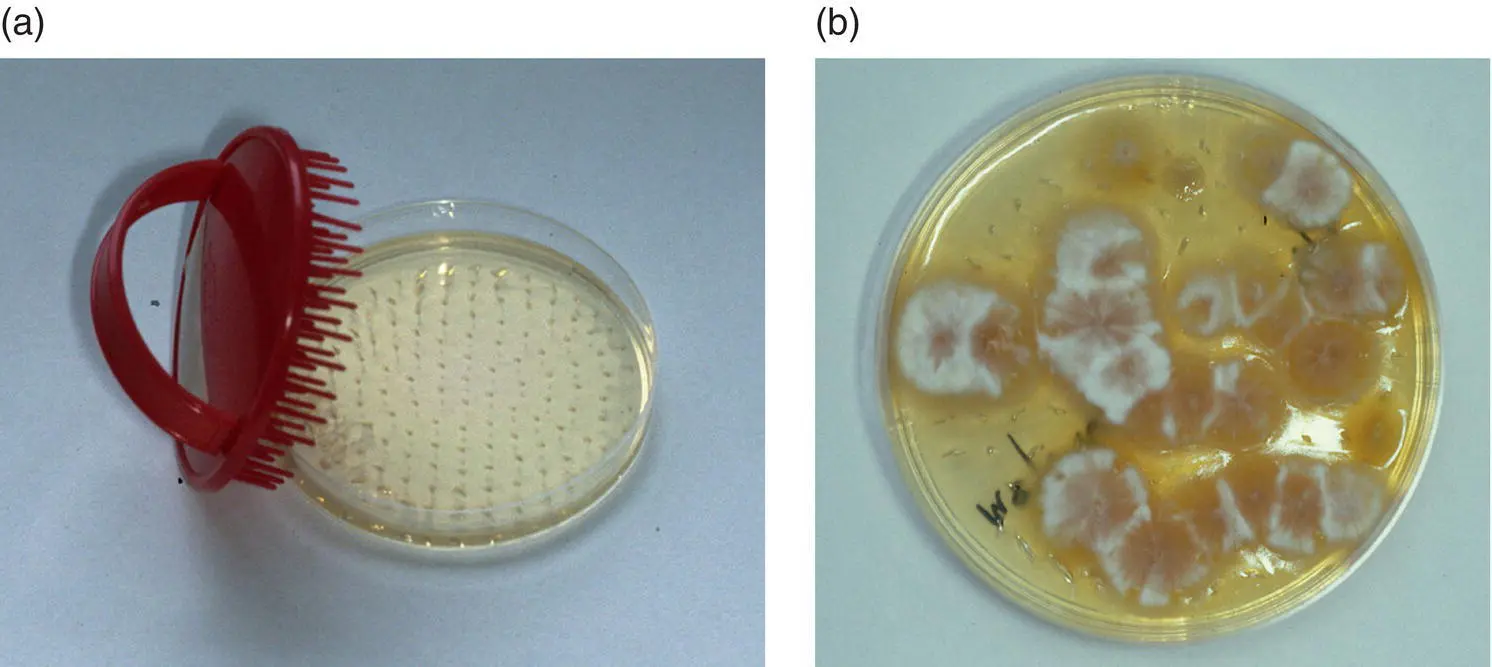
Figure 1.4 (a) A coarse‐toothed brush (e.g. 90 mm Denman scalp brush) facilitates sampling of large areas of skin and coat. The collected hair can be removed and examined, or the teeth may be embedded in a fungal culture medium as illustrated. (b) Here Microsporum canis has been isolated using this technique.
Skin scrapings can be performed for detection of external parasitic diseases such as chorioptic mange, larval stages of harvest mites, demodicosis (rare), or for dermatophyte culture and cytology.
If necessary, remove hair over areas to be sampled by careful clipping.
Use a wooden tongue depressor for superficial sampling or a large, curved scalpel blade if deeper samples are required, with the sharp edge blunted to reduce the risk of injuring the horse or operator.
Moisten the sample site or the collection tool with liquid paraffin (more useful for examination for mites), water or normal saline (for dermatophytes).
Gently scrape crusts, scales, and associated hair so that the material accumulates on the blade or tongue depressor. Transfer onto a microscope slide with more liquid paraffin, or with potassium hydroxide solution if collected in aqueous medium, which allows clearing of debris and easier identification of pathogens.
Deeper scrapings are needed for suspected demodicosis, deep enough to cause capillary ooze.
Sample several sites, collect plenty of material, and divide amongst several slides to make thin suspensions, which are quicker and easier to examine efficiently.
Surface adhesive tape samples
An alternative method for obtaining surface material, including Oxyuris equi eggs, surface‐living ectoparasites, hair fragments, exfoliated cellular material, and surface microorganisms for direct microscopical examination or after staining; it is less traumatic and avoids the risk of injury associated with skin scrapes. This technique is particularly useful for identification of chorioptic mange mites which are highly motile, but also allows detection of other pathogens including dermatophytes and yeasts.
A piece of clear adhesive tape (e.g. 3M Scotch Crystal, Sellotape Clear) is applied to the lesional area 3–4 times ( Figure 1.5).
Tape is applied (sticky side down) to a microscope slide over a drop of liquid paraffin for direct examination or over a drop of blue dye from a rapid Romanowsky‐type stain kit.
Excessive mounting medium or stain is removed by wiping with soft paper towel prior to microscopical examination.

Figure 1.5 Surface adhesive tape sampling.
From fresh, exudative, crusted, excoriated, or pustular lesions, a direct impression smear can be made for cytological examination and for microorganisms.
Press a glass slide against the concave undersurface of a removed exudative crust, or against the surface of a freshly exposed lesion.
For an intact pustule, gently break the overlying skin with a 25 g needle and press a clean glass slide to the ruptured lesion, or purulent material may be collected in the needle bevel and then transferred to the glass slide.
For lesions at sites where it is difficult to apply a slide directly, material can be collected with a dry swab and then rolled onto a glass slide.
Air‐dry the slide and store in a slide box prior to heatfixing (for Gram staining) or immersion in methanol (for Romanowsky‐type staining).
For older, crusted lesions this technique enables microscopical examination of dried exudate.
Representative sample of crust is placed on a glass slide with a few drops of normal saline.
Material is finely chopped and macerated with a scalpel blade.
Slide is left in a warm place for 20–30 min to allow rehydration of cellular material.
Any large clumps of debris are gently removed prior to thorough drying and heat fixing of the remaining suspension prior to staining with a rapid Romanowsky‐type stain kit.
Читать дальше














