Some atoms are prone to gaining electrons from outside sources, which results in them having a net negative charge. Other atoms are prone to losing electrons, resulting in a net positive charge. The resulting charge, positive or negative, is called the valence state (or valence charge) of an atom. Charged atoms are called ions; specifically, positively charged ions are called cations while negative ones are called anions ( Figure 1.2). The exchange (gain or loss) of electrons almost always occurs within the outermost portion of the electron cloud.
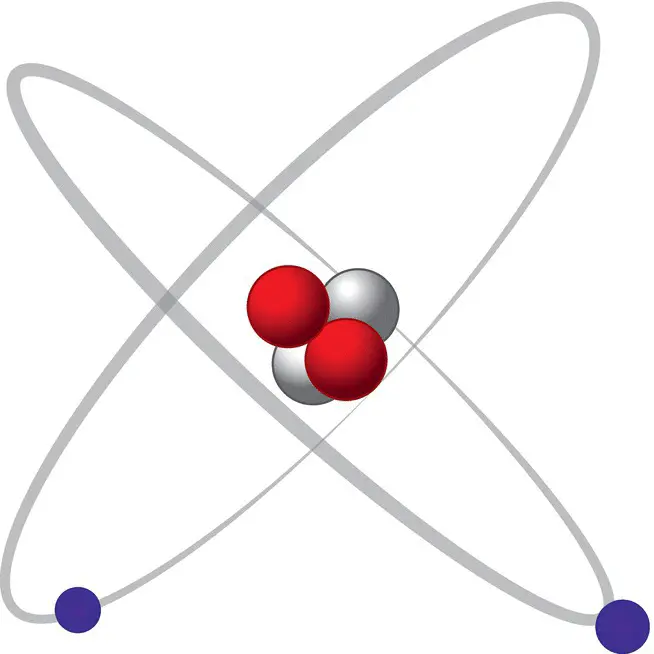
Figure 1.1 An atomic model of the element helium (He), with two protons, two neutrons, and two electrons.
The electron cloud of an ion can be estimated to be in the shape of a sphere and its size is defined by the distance from the center of the nucleus to the limit of the cloud. This is called the ionic radius, which is measured in units called Angstroms or Å. An Angstrom unit is very short – it is equal to one tenth of a nanometer. Note that a nanometer is 0.000000001 meter or 10 –9meter!
There are 92 naturally occurring elements out of a total 118 identified, each with its own symbol that acts as a shorthand notation. Some familiar elements and symbols are Au for gold, C for carbon, Ag for silver, and Pt for platinum. Element abbreviations start with a capital letter and if a second letter is present it will always be lowercase. Many elements are already part of our everyday vocabulary, such as oxygen (O), carbon (C), nitrogen (N), and potassium (K) but others are much more obscure, such as beryllium (Be), scandium (Sc), and rhodium (Rh).
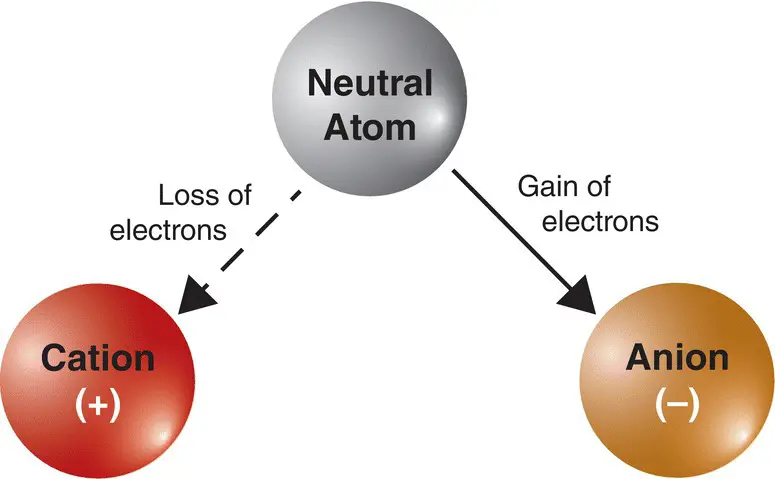
Figure 1.2 An atom can lose electrons and become a cation, a positively charged ion. If it gain electrons, it becomes an anion, a negatively charged ion.
A group is a column of elements in the periodic table ( Figure 1.3). Elements within a group have similar chemical behavior because of the similarity in the distribution of their electrons, especially in the valence (outermost) shell. The elements of the first group are called the alkali metals and tend to give up an electron, resulting in a characteristic +1 valence charge. A familiar element in this group is sodium (Na), part of the NaCl (table salt) molecule. Although hydrogen sits at the top of the column, it does not actually belong to the alkali metals group.
The elements of the second group are collectively called the alkaline earth metals. These elements usually lose two electrons, resulting in a characteristic +2 valence charge. Calcium (Ca) and magnesium (Mg), two of the important bone‐forming ingredients, are elements of this group.
The middle block of elements (ranging from Sc down and across to element 112, Cn) are called the transition metals. These elements can have variable valence charges, usually up to +4 but sometimes as high as +6. Note how the precious metals Cu (copper), Ag (silver), and Au (gold) are all Group 1B transition metals and thus share similar physical properties. The metals Ni (nickel), Pd (palladium), and Pt (platinum) are similarly related as Group VII elements. The transition elements often endow gemstones with their striking colors.
Elements classified as semi‐metals or other metals include aluminum (Al) and lead (Pb). The next group are the metalloids, including silicon (Si) and arsenic (As). Nonmetals include the biologically important elements carbon (C), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S).
The halogens occupy the seventeenth column and will almost always have a –1 charge. Familiar elements in this group are chlorine (Cl) and iodine (I). Elements on the far right are the noble gases, which do not combine with other elements. Notable gases in this group are helium (He) and neon (Ne). The two large blocks below the table are the Lanthanide and Actinide series elements.
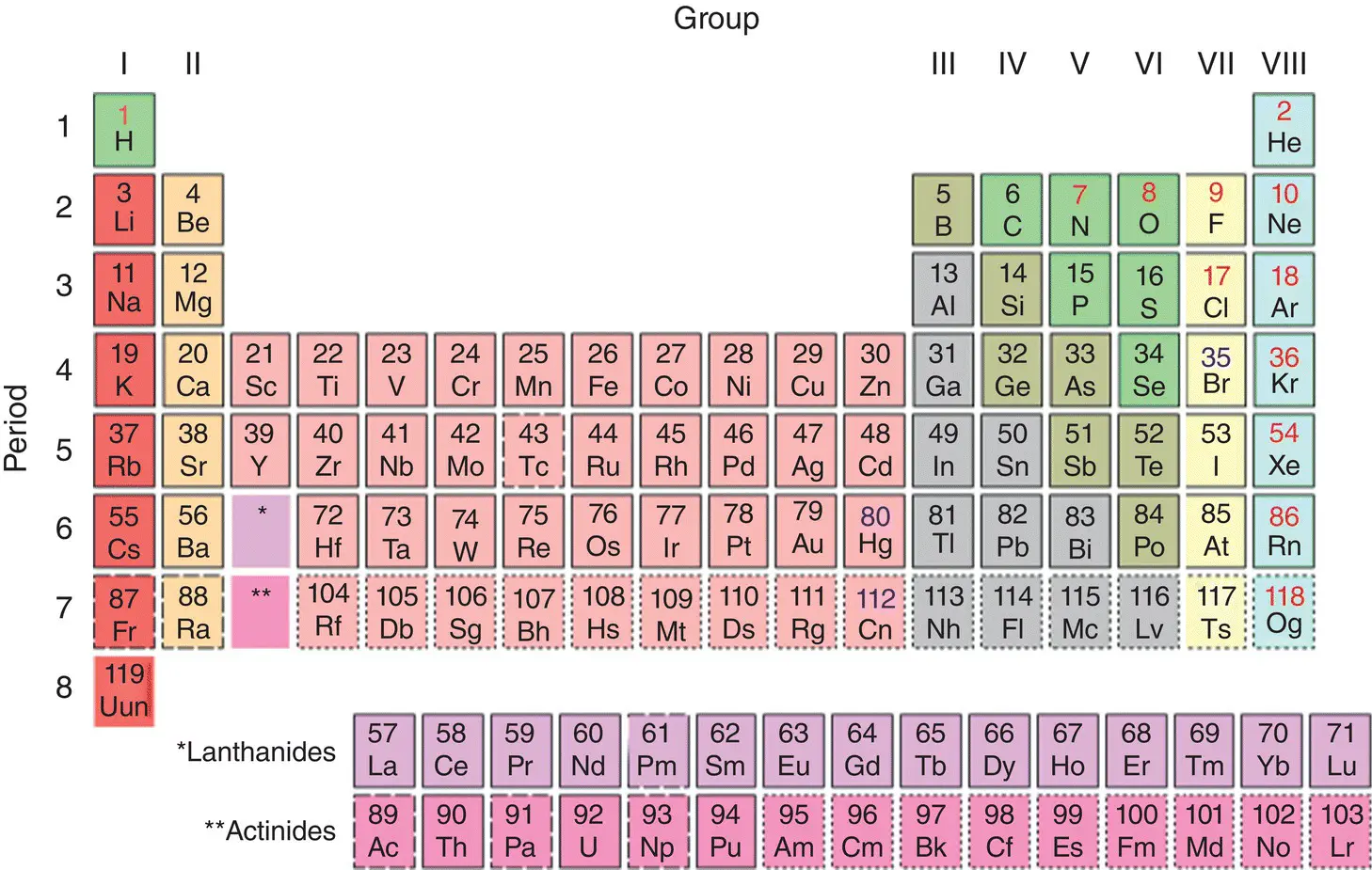
Figure 1.3 Periodic table of the elements with atomic numbers and chemical abbreviations. Dashed and dotted borders indicate the element is not naturally occurring.
1.3.6 Elemental Abundance in the Earth’s Crust
Although the periodic table appears to suggest that the elements are equally abundant and distributed proportionally on Earth, this is far from the case. The chemical composition of the Earth’s crust is in fact made up of eight dominant elements that comprise ~98.5%; all other elements combined make up the remaining ~1.5%. This distribution is shown in Table 1.2. Consequently, the bulk of the minerals commonly encountered have their base chemical formula closely associated with these eight elements.
The precious metal elements (e.g., Au, Pt, Ag, and Rh) occur very rarely in the Earth’s crust. Figure 1.4is a graphic showing the relative abundance of the elements (vertical axis) against their atomic number (horizontal axis). Note the highlighting of the top eight rock‐forming elements, the rarest metals, and the Rare Earth Elements (also known as the Lanthanide Series). Because of the large variability in abundance of elements, the vertical scale in Figure 1.4is logarithmic.
Table 1.2 Approximate abundance of dominant elements in the Earth’s crust. Data from Mason and Moore (1982).
| Element, Symbol |
Abundance in Earth’s crust (weight %) |
| Oxygen, O |
46.60 |
| Silicon, Si |
27.72 |
| Aluminum, Al |
8.13 |
| Iron, Fe |
5.00 |
| Calcium, Ca |
3.63 |
| Sodium, Na |
2.83 |
| Potassium, K |
2.59 |
| Magnesium, Mg |
2.09 |
| All others |
1.41 |
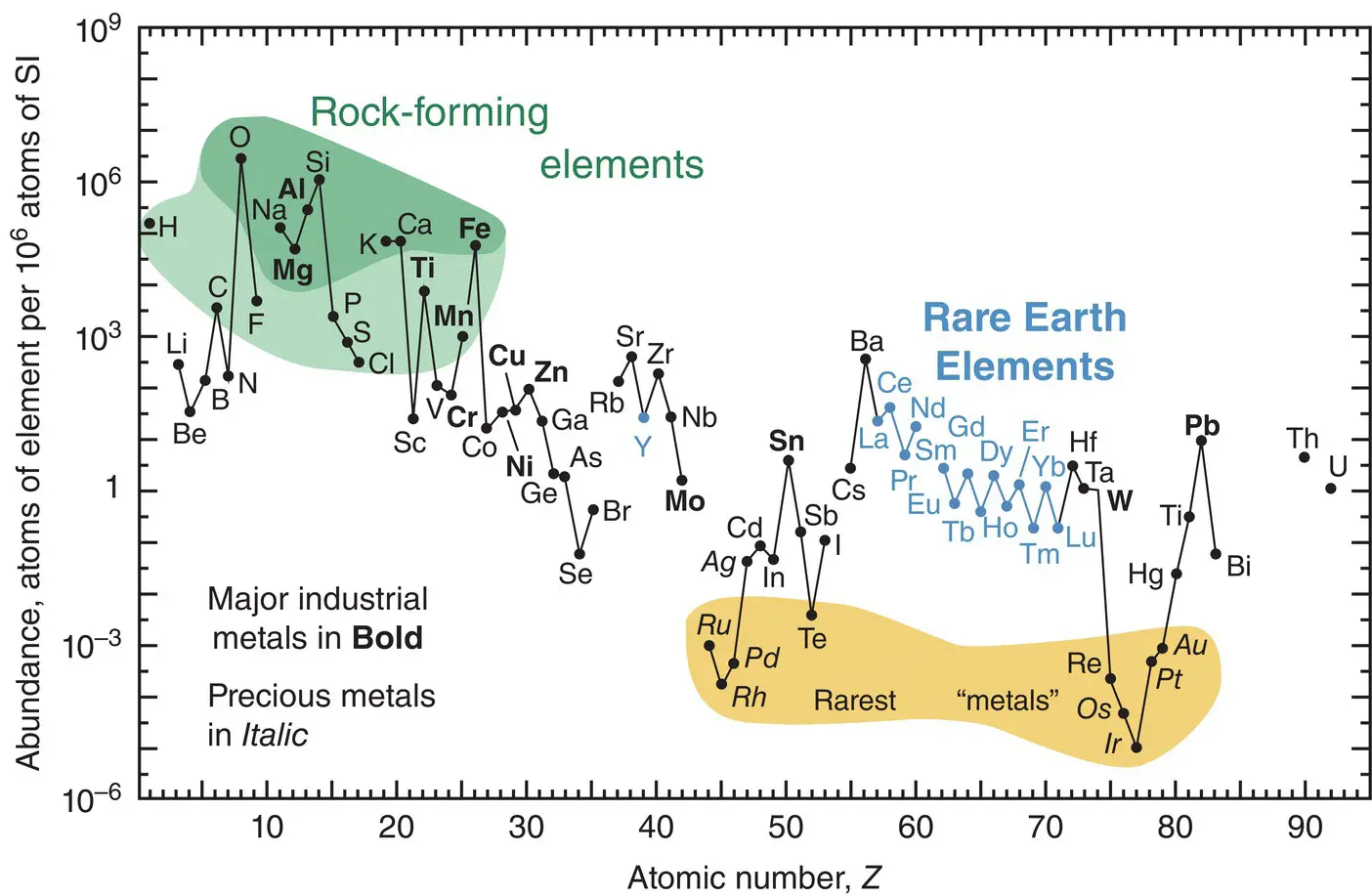
Figure 1.4 Relative abundance of the elements in the Earth's crust as compared to silicon atoms. Haxel et al. (2002) / U.S. Geological Survey / Public domain.
Most elements are generally very rare; their concentrations are therefore commonly reported in parts per million, or ppm. The value of “1 ppm” indicates that there will be one gram for every million grams of the material (i.e., 1 gram in every tonne). A value of 10,000 ppm is equivalent to 1% (10,000 parts for every million). The level of concentration in the Earth's upper crust for gold is approximately 0.002 ppm. This is the same as 2 parts per billion (ppb), meaning for every billion atoms counted, only two will be gold!
1.3.7 Compounds and Mixtures
Elements combine and interact through chemical bonds. When two or more elements join together they form a compound. As with an element, a compound is represented by symbols called chemical formula. Examples of common compounds and their formulae are water (H 2O), composed of hydrogen (H) and oxygen (O), and common table salt, (NaCl) composed of sodium (Na) and chlorine (Cl). Most gemstones are compounds, such as sapphire (Al 2O 3), composed of aluminum (Al) and oxygen, and emerald (Be 3Al 2Si 6O 18), composed of beryllium (Be), aluminum, silicon (Si), and oxygen.
Читать дальше
















