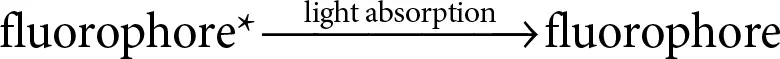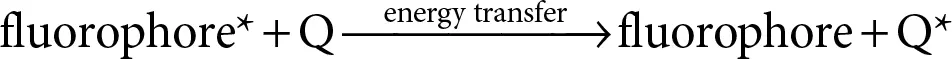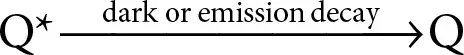2.5.1 Logic Gates Using Deoxyribozymes
It has been proved that deoxyribozymes, which is the DNA counterparts of ribozymes, can be used to develop logic gates and can solve simple computational problems [6]. Ribozymes, which are specific nucleic acid enzymes, exist in nature. These enzymes are structurally single stranded RNA molecules which have catalytic power and several other biological activities. On the other hand, deoxyribozymes, which are also single stranded structure, have to be synthesized in vitro . These enzymes generally catalyze the reactions of nucleic acid substrates, such as DNA-RNA ligation, RNA cleavage, and many more.
2.5.1.1 Catalytic Activity of Deoxyribozyme
One of the reactions that deozyribozyme catalyzes is to break the phosphodiester bond which cleaves the DNA or RNA strands. This reaction is commonly performed in the domain of DNA computing. In particular, the enzyme can be phosphodiesterase. By structural modification of this enzyme, the catalytic activity can be altered. For example, if a specific bonding domain is added to deoxyribozyme, then it can specifically bind to a particular complementary nucleic acid strand. These kinds of ligand nucleic acid strands strongly influence the catalytic activity of deoxyribozyme. The catalytic function of the enzyme can be either increased or inhibited by the association of the ligand strands. If the deoxyribozyme actively catalyzes the reactions, then it can cleave oligonucleotide in two shorter products which is shown in Figure 2.12. This reaction is used in the design of DNA logic gate. The generated shorter nucleic acid strands are the output signal of the gate. These signals can further be used as the input of another downstream gate and so on. Thus, using the gate structures and cleaving reactions a large DNA logic circuit can eventually be formed. The output signals of the larger circuit are also represented by short single stranded DNA strands which are tagged with fluorophore to read the signal.
The mechanism of cleaving nucleic acid strand is shown in Figure 2.12which is used to perform simple computations and logic operations. In the figure, the substrate strand, i.e., the strand which has to be cleaved is tagged by fluorophore at one end and quencher molecule at the other end. Quenching process decreases the fluorescence intensity of certain molecule. In the process of fluorophore quenching, first the fluorophore enters into excited state by absorbing light [7].

Figure 2.12 Catalytic activity of deoxyribozyme.
(2.19) 
If quencher is absent, then the fluorophore again returns to the ground state by the emission of light which causes the fluorescence.
(2.20) 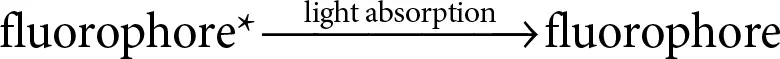
In presence of quencher, the excited fluorophore nonradiatively, i.e., without emitting light, transfers its energy to the quencher. This causes the quencher to enter into excited state, on the other hand, the fluorophore returns to its ground state.
(2.21) 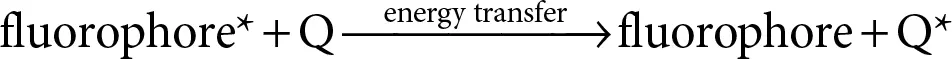
In the next step, the quencher returns to its ground state by releasing the absorbed energy. The release of energy can occur through emissive decay which causes fluorescence; or by dark quenching in which the energy releases non-radiatively, i.e., through molecular vibrations (heat).
(2.22) 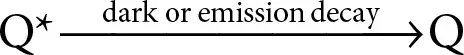
The fluorescence intensity increases proportionally as the distance between fluorophore and quencher increases.
In Figure 2.12, if substrate is cleaved, then the two short strands go apart which allow the fluorescence of the output signal. Otherwise, the fluorescence is suppressed by the quencher.
2.5.1.2 Controlling Deoxyribozyme Logic Gate
Deoxyribozyme can be either in active or in inactive state. This enzyme can be made switch sensitive to an input DNA strand by adding a stem-loop structure to the molecule. In the stem-loop structure, the two substrate binding regions are complementary to each other, thus these regions hybridize to each other to form the stem component. These formed structures inhibit the substrates to bind to its recognition site in deoxyribozyme.
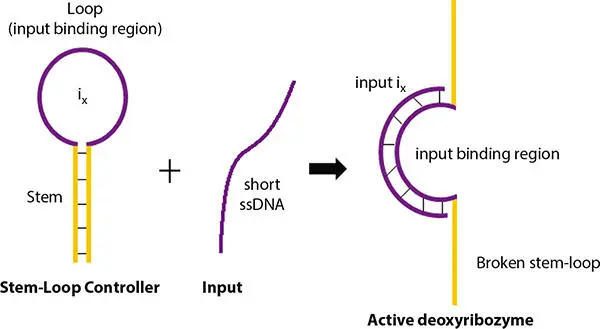
Figure 2.13 Mechanism to switch on deoxyribozyme logic gate.
The mechanism of controlling the catalytic activity of deoxyribozyme is shown in Figure 2.13. Deoxyribozyme logic gate can be switched on by addition of a single stranded short input DNA strand. This input oligonucleotide binds to the complementary single stranded loop region of deoxyribozyme. The hybridization leads to the destabilization of the stem-loop structure of deoxyribozyme. As a result, the stem-loop module opens up which permits the substrate to bind with deoxyribozyme. Thus, it can be concluded that the addition of the input oligonucleotides leads to the conformation change of the stemloop region of the enzyme which causes it is the catalytic activity. Following this method, the DNA logic gate can be switched on. This mechanism can also be applied for large DNA logic circuit. In this case, many different DNA oligonucleotides selectively bind to the specific corresponding stem-loop regions of deoxyribozyme. Thus, the activation of the logic circuit can be controlled by adding all inputs and enzymes in the same solution.
Now, in the next subsections, we will discuss the basic logic gates that can be constructed by using deoxyribozyme [6].
Deoxyribozyme logic gate works as YES gate if it is modified by including a single stemloop region which regulates the binding of substrate, as explained in the previous subsection. The substrate cannot bind to deoxyribozyme if the stem-loop is in closed form. As a result, the enzyme is inactivated as no output can be produced. Addition of input single stranded DNA sequence causes the hybridization of it to the loop region and breakage of stem-loop structure. This conformational change activates the enzyme. Now, the substrate binding regions are free to hybridize which causes the cleavage of the substrate. Now, the output DNA signal can be produced. This mechanism is shown in Figure 2.14, where a single input ix activates the YES x logic gate. Table 2.4represents the truth table for YES gate.
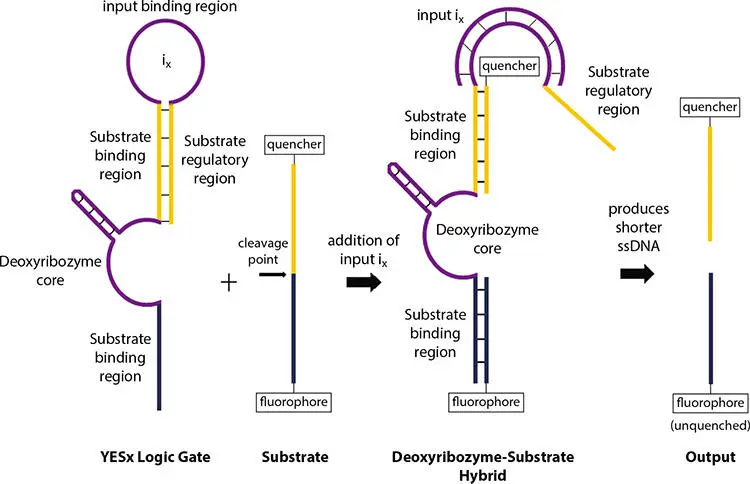
Figure 2.14 Mechanism of YES gate.
Table 2.4 Truth table for YES gate.
| Input (ix) |
Output (iy) |
| 0 |
0 |
| 1 |
1 |
Читать дальше