John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
This website includes:
Figures and Tables from the book
Appendices and additional resources, including detailed descriptions of major rock-forming minerals and keys for identifying minerals using macroscopic and/or optical methods
Chapter 1 Earth materials and the geosphere
1 1.1 Earth materials
2 1.2 Minerals and mineraloids
3 1.3 The geosphere
4 1.4 Detailed model of the geosphere
5 1.5 Global tectonics
6 1.6 Hotspots and mantle convection
1.1 EARTH MATERIALS
This book concerns the nature, origin, evolution, and significance of Earth materials. Earth is composed of a variety of naturally occurring and synthetic materials whose composition can be expressed in many ways. These include their chemical, mineral, and rock composition. In simple terms, atoms combine to form minerals and minerals combine to form rocks. Discussion of the relationships between atoms, minerals, and rocks is fundamental to an understanding of Earth materials, their properties, and the processes that produce them.
1.2 MINERALS AND MINERALOIDS
The term mineralis used in a number of ways. For example, the chemical elements, such as calcium, iron, and potassium, listed on your breakfast cereal box, your bottle of vitamin supplements or your bag of fertilizer are called minerals. Coal, oil, and gas are referred to as mineral resources. All of these fall under a broad use of the term mineral. In a stricter sense used by many, but not all geologists, minerals are defined by the following properties:
1 Minerals are solid, so do not include liquids and gases. Minerals are solid because the atoms in them are held together in fixed positions by forces called chemical bonds ( Chapter 2).
2 Minerals are naturally occurring, meaning that they occur naturally within the Earth. This definition excludes synthetic solids that are produced only by technologies in laboratories or factories. It does include solid Earth materials that are produced by both natural and synthetic processes, such as natural and synthetic diamonds and the solid materials synthesized in high temperature and high pressure laboratory experiments that are thought to be analogous to real minerals that occur only in the deep interior of Earth.
3 Each mineral species has a specific chemical composition which may vary only within well‐defined limits; that is to say that each mineral possesses a chemical composition that can be expressed by a chemical formula. An example is common table salt or halite which is composed of sodium and chlorine atoms in a 1 : 1 ratio (NaCl). Chemical compositions may vary within well‐defined limits because minerals incorporate impurities, have atoms missing, or otherwise vary from their ideal compositions. In addition some types of atoms may substitute freely for one another when a mineral forms generating a well‐defined range of chemical compositions. For example, magnesium (Mg) and iron (Fe) may substitute freely for one another in the mineral olivine whose composition is expressed as (Mg,Fe)2SiO4. The parentheses are used to indicate the variable amounts of Mg and Fe that may substitute for each other in olivine group minerals ( Chapter 3).
4 Every mineral species possesses a long‐range, geometric arrangement of constituent atoms or ions. This implies that the atoms in minerals are not randomly arranged. Instead minerals crystallize in geometric patterns so that the same pattern is repeated throughout the mineral. In this sense, minerals are like three‐dimensional wall paper. A basic pattern of atoms, a motif, is repeated systematically to produce the entire geometric design. This long range pattern of atoms characteristic of each mineral species is called its crystal structure. All materials that possess geometric crystal structures are crystalline materials. They are minerals, in the narrow sense, if they are naturally occurring, inorganic solids with a well‐defined chemical composition. Solid materials that lack a long‐range crystal structure are amorphous materials, where amorphous means without form and without a long‐range geometric order.
Many would add a fifth property that requires minerals to sometimes be formed by inorganic processes. It is certainly true that the vast majority of minerals conform to this property and that the vast majority of organically formed crystalline solids are not considered to be minerals. However, many solid Earth materials that form by both inorganic and organic processes are considered minerals, especially if they are important constituents of naturally formed rocks. For example, the mineral calcite is also precipitated as shell material by organisms such as clams, snails, and corals and is the major constituent of the rock limestone ( Chapter 14).
Over 5500 minerals have been discovered to date ( www.mindat.com) and each is distinguished by a unique combination of chemical composition and crystal structure. Strictly speaking, naturally occurring, solid materials that lack one of the properties described above are commonly referred to as mineraloids. Common examples include amorphousmaterials such as volcanic glass in which the atoms lack long‐range order and amber or ivory which are formed only by organic processes.
1.2.1 Rocks
Earth is largely composed of various types of rock. A rock is an aggregate of mineral crystals and/or mineraloids. Scarce monominerallicrocks consist of multiple crystals of a single mineral. Examples include the sedimentary rock quartz sandstone which may consist entirely of quartz grains held together by quartz cement and the igneous rock dunite which can consist entirely of olivine crystals. The vast majority of rocks are polyminerallic; they are composed of many types of mineral crystals. For example, granite commonly contains quartz, potassium feldspar, plagioclase, hornblende and/or biotite, and various other minerals in small amounts.
Mineral composition is one of the major defining characteristics of rocks. Rock textures and structures are also important defining characteristics. It is not surprising that the number of rock types is very large indeed, given the large number of different minerals that occur in nature, the different conditions under which they form, and the different proportions in which they can combine to form aggregates with various textures and structures. Helping students to understand the properties, classification, origin, and significance of minerals and rocks is the major emphasis of this text.
1.3 THE GEOSPHERE
Earth materials can occur anywhere on or within the geosphere, the portion the Earth from its surface to its center, whose radius is approximately 6370 km ( Figure 1.1). In static standard models of the geosphere, Earth is depicted with a number of roughly concentric layers. Some of these layers are distinguished primarily on the basis of differences in composition and others by differences in their state or mechanical properties. These two characteristics by which the internal layers of Earth are distinguished are not totally independent, because differences in chemical, mineralogical and/or rock composition influence mechanical properties.
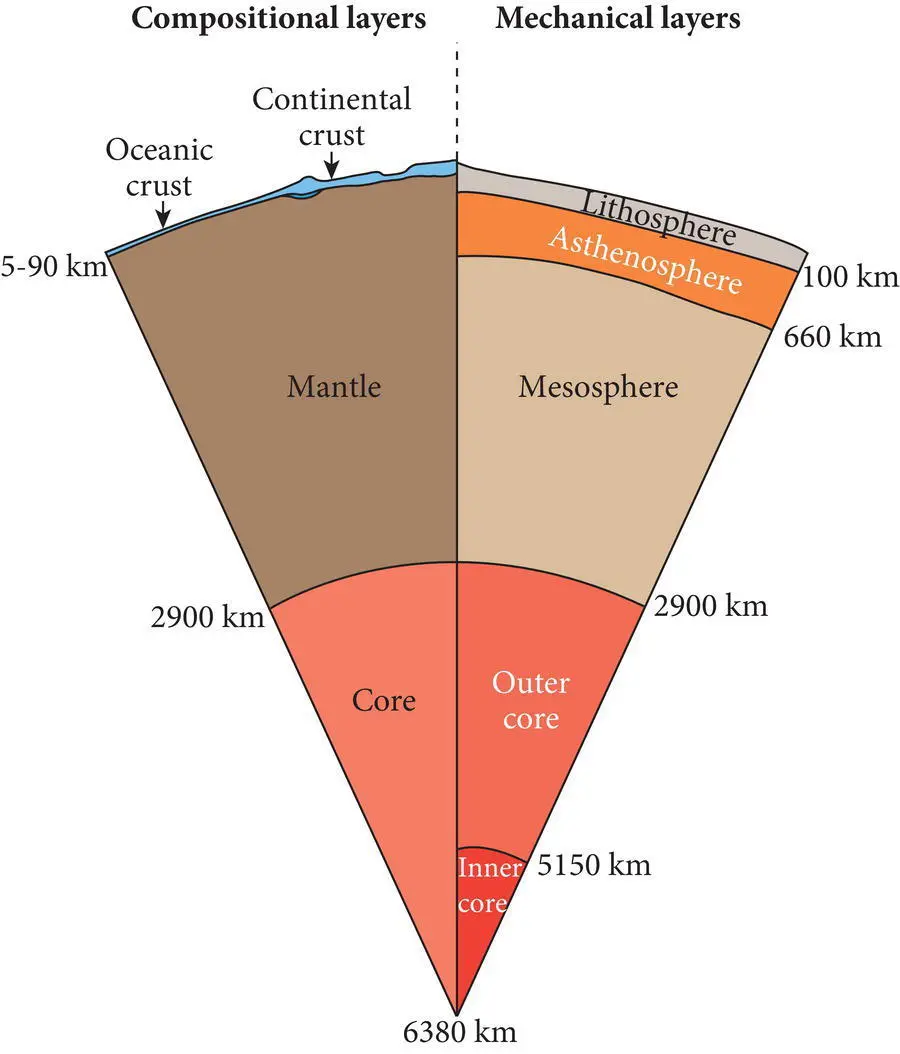
Figure 1.1 Standard cross‐section model of the geosphere. Major compositional layers are shown on the left: core (red shade), mantle (brown shade), and continental and oceanic crust (blue shades). Major mechanical layers are shown on the right: inner core and outer core (red shades), lithosphere (light brown), mesosphere (dark brown), and asthenosphere (burnt orange).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












