Contemporary Accounts in Drug Discovery and Development
Здесь есть возможность читать онлайн «Contemporary Accounts in Drug Discovery and Development» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Contemporary Accounts in Drug Discovery and Development
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Contemporary Accounts in Drug Discovery and Development: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Contemporary Accounts in Drug Discovery and Development»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A useful guide for medicinal chemists and pharmaceutical scientists Contemporary Accounts in Drug Discovery and Development
Contemporary Accounts in Drug Discovery and Development
Contemporary Accounts in Drug Discovery and Development
Contemporary Accounts in Drug Discovery and Development — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Contemporary Accounts in Drug Discovery and Development», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Table 3.7 Population pharmacokinetic model based steady‐state geometric mean (CV%) plasma pharmacokinetic parameters of vericiguat 2.5, 5, or 10 mg in heart failure patients ( N = 2321).
| PK parameters | 2.5 mg | 5 mg | 10 mg |
|---|---|---|---|
| C max(μg/l) | 120 (29.0) | 201 (29.0) | 350 (29.0) |
| AUC (μg•h/l) | 2300 (33.9) | 3850 (33.9) | 6680 (33.9) |
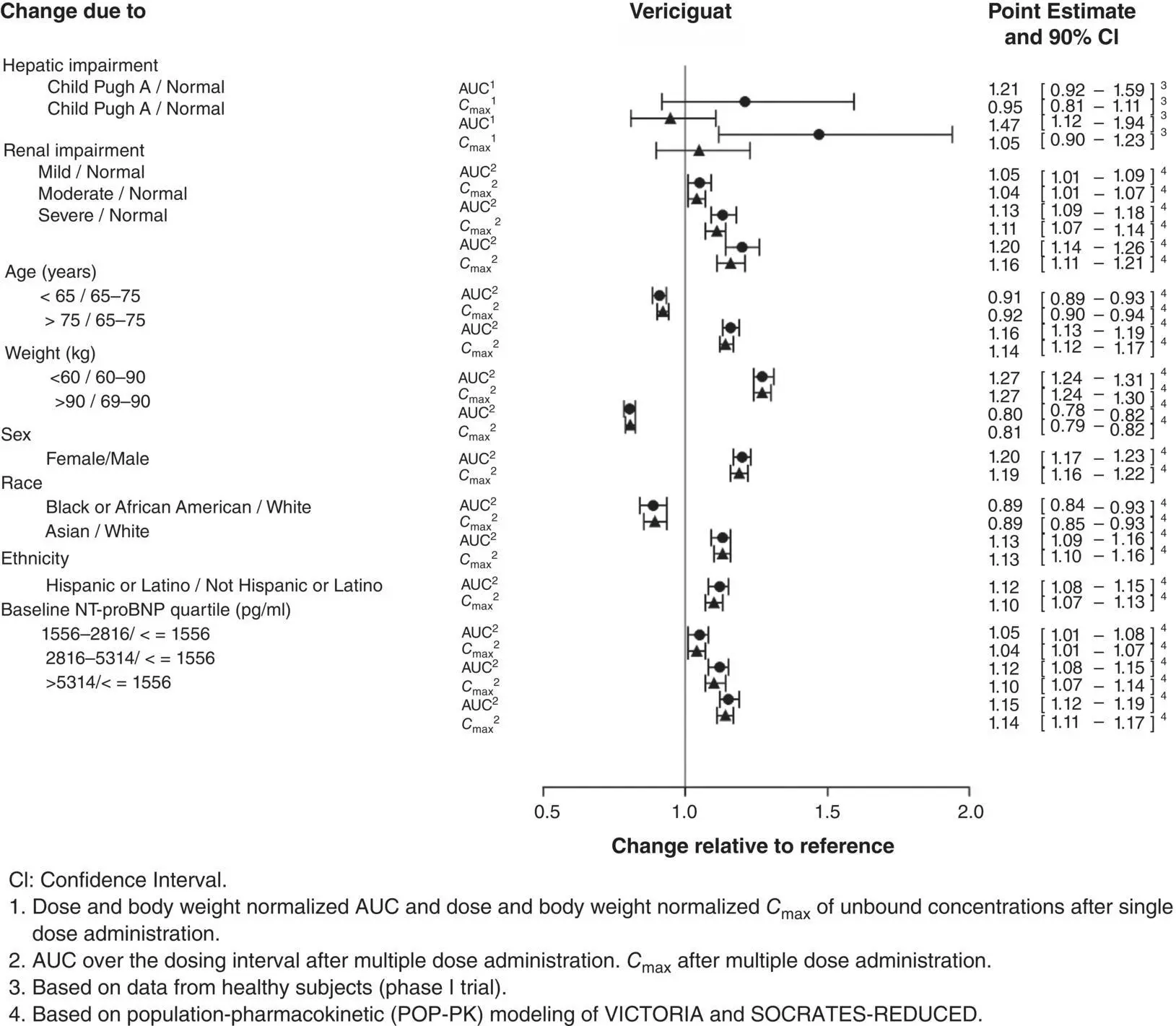
Figure 3.7 Pharmacokinetics of vericiguat in special populations.
3.6.2.6 Special Populations
Based on a population pharmacokinetic analysis, age, gender, ethnicity, race, and baseline NT‐proBNP have not been identified as significant co‐variates and thus do not have a clinically meaningful effect on the PK of vericiguat in HFrEF patients. The effect of body weight on vericiguat exposure was not clinically meaningful. No clinically relevant increase in exposure was observed for subjects with mild hepatic impairment (Child‐Pugh A and B). The PK of vericiguat have not been studied in patients with severe hepatic impairment (Child‐Pugh C) ( Figure 3.7).
3.6.2.7 Drug Interactions
In vitro studies indicate that vericiguat and its N‐glucuronide are neither inhibitors of major CYP isoforms (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4) or UGT isoforms (UGT1A1, 1A4, 1A6, 1A9, 2B4, and 2B7), nor inducers of CYP1A2, 2B6, and 3A4, at clinically relevant concentrations.
Vericiguat is a substrate of P‐glycoprotein (P‐gp) and breast cancer resistance protein (BCRP) transporters and is not a substrate of organic cation transporter (OCT1), or organic anion transporting polypeptides (OATP1B1 and OATP1B3). Vericiguat and its N‐glucuronide are not inhibitors of drug transporters, including P‐gp, BCRP, BSEP, OATP1B1/1B3, OAT1, OAT3, OCT1, OCT2, MATE1, and MATE2K, at clinically relevant concentrations. Overall, these data indicate that the administration of vericiguat is unlikely to affect the PK of concurrently administered medications that are substrates of these enzymes or transporters.
3.6.2.8 In vivo Assessment of Drug Interactions
Effects of Other Drugs on the Pharmacokinetics of Vericiguat
The effects of coadministered drugs on the PK of vericiguat have been assessed in 10 clinical drug–drug interaction studies. Overall, there was no clinically relevant effect on vericiguat PK with coadministration of drugs increasing gastric pH (e.g. proton pump inhibitors, H2‐receptor antagonists, antacids) in HF patients; or with coadministration of mefenamic acid, ketoconazole, rifampicin, digoxin, warfarin, aspirin, sildenafil, or the combination of sacubitril/valsartan in healthy subjects [43]. There was no clinically relevant effect on vericiguat PK with coadministration of atazanavir based on physiologically based pharmacokinetic (PBPK) modeling. The results are summarized in Figure 3.8.
These data indicate that no dose adjustment of vericiguat is recommended when coadministered with commonly prescribed medicinal products [44].
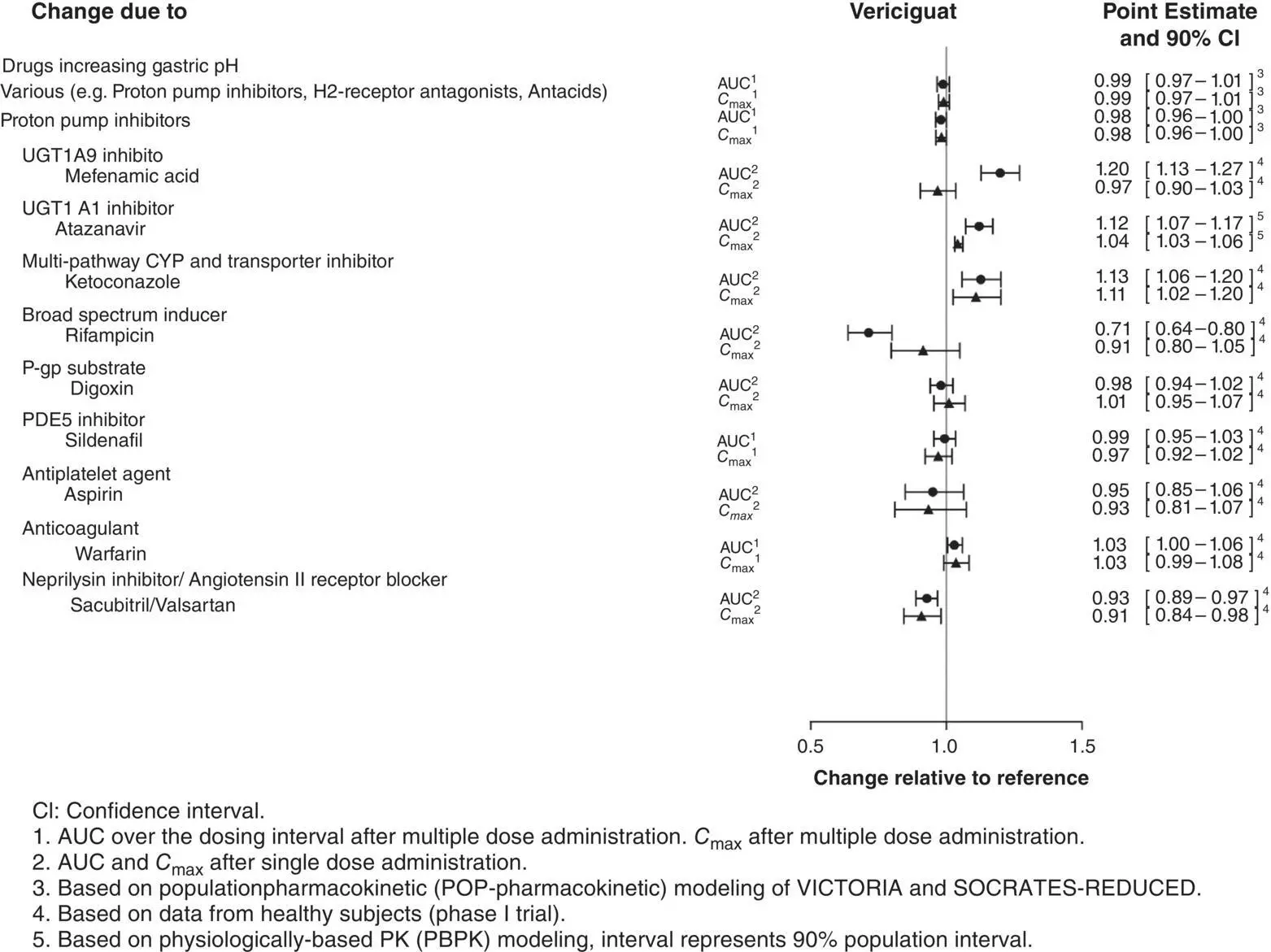
Figure 3.8 Effects of other drugs on the pharmacokinetics of vericiguat.
Effects of Vericiguat on the Pharmacokinetics of Other Drugs
The effects of vericiguat on the PK of coadministered drugs have also been assessed in clinical drug–drug interaction studies [43, 45]: vericiguat had no clinically relevant effect on the PK of midazolam, digoxin, warfarin, sildenafil, and the combination of sacubitril/valsartan when coadministered in healthy subjects ( Figure 3.9).
3.6.3 Pharmacodynamic Interactions
The effects of vericiguat on the PD of coadministered drugs have also been assessed in 6 clinical drug–drug interaction studies: vericiguat had no clinically relevant effect on the PD of aspirin, warfarin, sildenafil, and the combination of sacubitril/valsartan when coadministered in healthy subjects; or on the pharmacodynamics of organic nitrates when coadministered in patients with coronary artery disease [43, 45].
3.6.4 Vericiguat Phase 2 and Phase 3 studies in HFrEF patients
The transition from healthy volunteers to HFrEF patients investigated in SOCRATES‐REDUCED was supported by a modeling‐based bridging approach using clinical data from the sGC stimulators riociguat and nelociguat (BAY 60‐4552) replacing a standard proof‐of‐concept study and enabling a direct transition into Phase 2b [46].
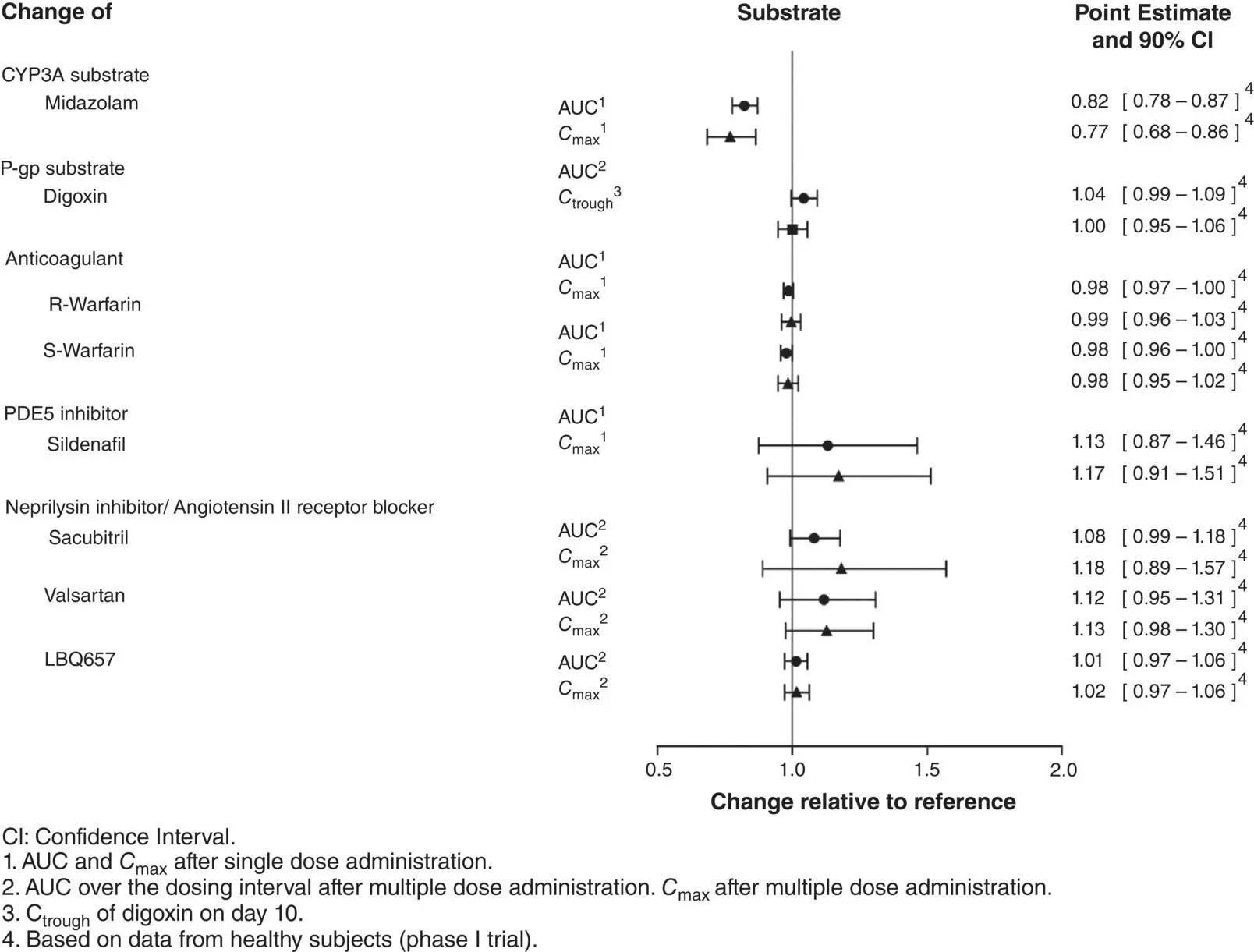
Figure 3.9 Effects of vericiguat on the pharmacokinetics of other drugs [44].
The SOCRATES program was designed to study in two parallel dose‐ranging trials vericiguat at titrated dosing schemes up to 10 mg once per day in the highest target dose arm over 12 weeks in patients with stabilized worsening chronic HFrEF (SOCRATES‐REDUCED) and in patients with stabilized worsening chronic HFpEF (SOCRATES‐PRESERVED). Based on insights from the riociguat LEPHT study [25, 26, 46], patients with symptomatic worsening chronic HF were enrolled irrespective of documented elevations in pulmonary artery pressure. Since very high mortality and morbidity rates persist in patients who experience HF events despite receiving optimal medical therapy, these studies specifically enrolled patients within a one month window after a preceding HF hospitalization, or alternatively after a HF event that had to be treated with i.v. diuretics in an outpatient setting [47]. Rather than transient management with additional i.v. therapy, long‐term treatment with an oral sGC stimulator was deemed to carry promise [48] to meet this medical need in patients suffering from HF events despite available therapies [49].
Primary endpoint in SOCRATES‐REDUCED was reduction in NT‐proBNP at 12 weeks, and the study was powered to detect a decrease in the 10 mg arm as previously observed in patients with HFrEF and concomitant PH in the 2 mg riociguat target dose arm of the LEPHT study [47]. Although the primary analysis of the pooled three higher dose arms did not reach statistical significance, the pre‐specified secondary analysis of the primary endpoint NT‐proBNP comparing the individual dose arms versus placebo showed a statistically and clinically significant reduction in NT‐proBNP in the 10 mg target dose arm at 12 weeks [50]. Based upon the established association of short‐term NT‐proBNP lowering with long‐term decreases in clinical events in patients with HFrEF, the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) phase 3 event‐driven outcome trial was designed to study whether vericiguat at a target dose of 10 mg once daily reduces mortality and morbidity in patients with worsening chronic HFrEF [18]. Different from other previous trials in patients with stable HFrEF, patients enrolled in the VICTORIA trial represent a broadly generalizable high‐risk population suffering from worsening chronic HFrEF despite very good HF therapy [51].
In parallel with the development in HFrEF, vericiguat was also studied in patients with worsening chronic HFpEF. Conducted back‐to‐back with the phase 2 HFrEF study, the SOCRATES‐PRESERVED trial enrolled patients within one month after an HF event who had a preserved ejection fraction (LVEF >45%) [47]. Opposed to the clinically meaningful reduction observed in patients with HFrEF in SOCRATES‐REDUCED, the same dose and duration of treatment with vericiguat did not reduce NT‐proBNP in patients with HFpEF, and changes in the other primary endpoint, left atrial volume, did not differ between treatment arms [52]. However, exploratory analyses showed an improved health status assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) and QoL measured with the EQ‐5D in patients treated with vericiguat [53]. Therefore, the phase 2b study VITALITY‐HFpEF has been designed to determine the efficacy and safety of vericiguat on QoL and exercise tolerance in patients with HFpEF [54], and results of this study showed that vericiguat did not improve the physical limitation score (KCCQ PLS) at 24 weeks [55].
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Contemporary Accounts in Drug Discovery and Development»
Представляем Вашему вниманию похожие книги на «Contemporary Accounts in Drug Discovery and Development» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Contemporary Accounts in Drug Discovery and Development» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












