Contemporary Accounts in Drug Discovery and Development
Здесь есть возможность читать онлайн «Contemporary Accounts in Drug Discovery and Development» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Contemporary Accounts in Drug Discovery and Development
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Contemporary Accounts in Drug Discovery and Development: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Contemporary Accounts in Drug Discovery and Development»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
A useful guide for medicinal chemists and pharmaceutical scientists Contemporary Accounts in Drug Discovery and Development
Contemporary Accounts in Drug Discovery and Development
Contemporary Accounts in Drug Discovery and Development
Contemporary Accounts in Drug Discovery and Development — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Contemporary Accounts in Drug Discovery and Development», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Upon the successful completion of phase 2 studies with the sGC stimulator riociguat in patients with pulmonary artery hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH), we hypothesized that direct sGC stimulation may also benefit patients in whom pulmonary hypertension was associated with left ventricular dysfunction ( Figure 3.4) [25]. Therefore, the LEft ventricular systolic dysfunction associated with Pulmonary Hypertension riociguat Trial (LEPHT) was conducted in parallel with the phase 3 studies PATENT and CHEST in PAH and in CTEPH, respectively. We tested the hypothesis that direct sGC stimulation with riociguat would reduce elevated mean pulmonary artery pressures (PAPs; inclusion criterion: above 25 mmHg) in patients suffering from heart failure with reduced ejection fraction (HFrEF) who were symptomatic despite optimized medical therapy at a stable dose regimen for >30 days before randomization. Although the primary endpoint reduction in PAPmean upon 16 weeks of treatment with riociguat compared with placebo both added to standard of care was not met, consistent dose‐dependent hemodynamic improvements were found in invasive hemodynamic measurements [26]. These included balanced reductions in both systemic and pulmonary resistance, which were associated with significant increases in cardiac index and stroke volume at the highest tested dose regimen, and a parallel decrease in N‐terminal pro–B‐type natriuretic peptide (NT‐proBNP) as marker of cardiac wall stress. Riociguat titration up to 2 mg was well tolerated, and the observed dose‐dependent hemodynamic improvements did not come at the expense of an increase in heart rate (HR) or decreases in blood pressure (BP) at 16 weeks of treatment.
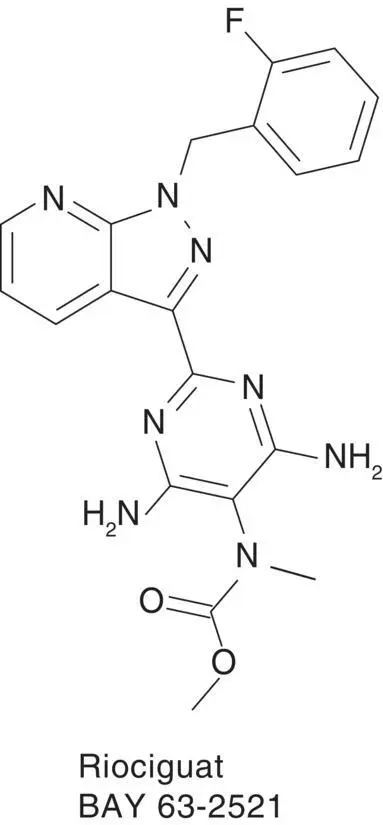
Figure 3.4 sGC stimulator riociguat.
Mechanistically, we postulated that balanced reductions in pulmonary and systemic resistances may facilitate unloading of the dysfunctional left ventricle, improving CV performance and flow without adverse compromise in BP. However, as patients with HFrEF in the LEPHT study were only eligible with elevated PAPmean including both pre‐ and post‐capillary pulmonary hypertension (PH), we considered whether the observed improvements were dependent on reductions in the precapillary component of PH in patients with high baseline pulmonary vascular resistance (PVR), or more generalizable resulting from decongestion addressing the post‐capillary passive PH component that occurs secondary to backward failure of the left ventricle. Subgroup analyses presented at the 18th Annual Scientific Meeting of the HFSA 2014 indicated that riociguat improved pulmonary capillary wedge pressure (PCWP), pulmonary artery compliance (PAC), stroke volume (SV), and quality of life (QoL) to a greater extent in patients with HFrEF, secondary PH, and low baseline PVR than in those with high baseline PVR ( Table 3.1[27]).
Since the LEPHT study was confined to patients with PH associated with left ventricular dysfunction and did not meet its primary endpoint, it was unclear at that time whether a benefit of direct sGC stimulation may be observed in patients with HFrEF and if this would be observed irrespective of the documented presence of secondary PH. Application of riociguat in other CV indications is limited by its short half‐life, necessitating a three times daily dosing regimen. In our efforts to further optimize the compound class, we have uncovered interesting structure–activity relationships and were able to decrease oxidative metabolism significantly. These studies resulted in the discovery of once daily sGC stimulator vericiguat (BAY 1021189; MK‐1242) that we decided to develop for chronic HF (see Section 3.6).
Table 3.1 Hemodynamic changes and QoL at 16 weeks in patients with high vs low PVR at baseline.
| PVR ≤240 dyn·s/cm 5 | PVR >240 dyn·s/cm 5 | Inter‐ action p ‐value | |||
|---|---|---|---|---|---|
| Parameter a | n b | LS mean diff. (95% CI)Riociguat 2 mg vs placebo | n b | LS mean diff. (95% CI)Riociguat 2 mg vs placebo | |
| SVR (dyn·s/cm 5) | 21/25 | −276 (−459 to −93) | 32/31 | −210 (−386 to −34) | 0.6 |
| SBP (mmHg) | 21/25 | −0.96 (−9.5 to 7.6) | 32/31 | +1.4 (−5.2 to 7.9) | 0.7 |
| Cardiac index (l/min/m 2) | 22/25 | +0.6 (0.3 to 0.9) | 32/31 | +0.2 (−0.02 to 0.4) | 0.03 |
| Stroke volume (ml) | 22/25 | +17 (7 to 28) | 32/31 | +7 (−1 to 14) | 0.07 |
| PCWP (mmHg) | 22/25 | −6.55 (−10.65 to −2.44) | 31/31 | +1.18 (−2.47 to 4.83) | 0.005 |
| mPAP (mmHg) | 22/25 | −8.8 (−13.3 to −4.3) | 32/31 | +1.1 (−3.4 to 5.6) | 0.002 |
| PAC (ml/mmHg) | 22/25 | +1.7 (0.9 to 2.6) | 32/30 | +0.4 (−0.2 to 1.0) | <0.005 |
| MLHF QoL total score | 27/30 | −12 (−21 to −3) | 40/39 | −6 (−12 to 0.2) | 0.3 |
aPer‐protocol population used for hemodynamics; ITT used for QoL. Estimates and CIs are based on an ANCOVA model with baseline value, treatment effect and region as fixed effects. p ‐value is based on an ANCOVA model with baseline value, treatment effect, region and subgroup as fixed effects and interaction of subgroup and treatment (placebo and riociguat 2 mg).
bRiociguat 2 mg/placebo [27].
3.3 Medicinal Chemistry Program
Optimization strategy.The goal of this medicinal chemistry program resulting in vericiguat 2was to identify an orally bioavailable sGC stimulator with a longer duration of action than riociguat 1. In order to support a profile allowing for once daily oral dosing, the strategy is to reduce oxidative metabolism which would also lower drug–drug interaction potentials. Riociguat is a very potent sGC stimulator in vitro and in vivo ; however, it has a moderate half‐life in different animal species [28] and this pharmacokinetic profile translated into a three times daily dosing regimen in patients. N ‐demethylation to compound 10appears to be the main biotransformation pathway of riociguat as described by Gnoth et al. [29] in 2015. This step is mainly catalyzed by CYP1A1, but also by CYP3A4, CYP3A5, and CYP2J2 [29–35]. Our optimization strategy was aiming for further optimizing the metabolic stability of riociguat and hence reducing blood clearance in order to achieve a longer half‐life. We began by varying the substituents on the 5‐carbamoyl residue on the pyrimidine ring, aiming to achieve metabolically more stable derivatives while maintaining good potency. In a second optimization step, we later focused on variations of the central pyrazolopyridine scaffold.
SAR and DMPK optimization.First, the SAR of a series of novel N‐substituted methyl carbamates 1, 3– 9was explored using a cGMP formation assay with sGC‐overexpressing Chinese hamster ovary (CHO) cells [36]. In addition, the metabolic stability of the analogs was assessed in vitro by incubation with rat hepatocytes. Initially, different carbamate N‐substituents were synthesized with the aim of achieving higher metabolic stability than 1while maintaining high potency ( Table 3.2). Increasing steric bulk (e.g. ethyl 3) or the introduction of polarity (e.g. a hydroxyethyl functionality, as in 4) led to a significant loss of potency relative to 1, along with decreased metabolic stability. Also, the introduction of fluorine atoms at the terminal position of the N‐substituent, a common strategy to block metabolism, was not met by success. Interestingly, both the 2,2‐difluoroethyl and 2,2,2‐trifluoroethyl derivatives, 5and 6, exhibited high potency in the cGMP assay but unfortunately had a 2‐ to 4.5‐fold higher clearance in rat hepatocytes. N ‐fluorobenzyl substitution revealed that the ortho ‐fluoro derivative 7is significantly more potent (MEC = 0.1 μM) than the meta and para isomers, 8and 9(MEC = 0.2 and 0.7 μM, respectively). Nevertheless, all the benzylic derivatives were characterized by high clearance in rat hepatocytes. These results and the testing of many further derivatives (data not shown) suggested that improving the metabolic stability by altering the N‐substitution might be very difficult to achieve. As a consequence, optimization efforts were focused on the main metabolite of riociguat, the N ‐desmethyl derivative 10. This compound is less potent than 1but displayed a somewhat higher metabolic stability in the rat hepatocyte assay, with a clearance of 0.1 l/h/kg. When tested in rats (i.v. dosing), compound 10had insufficient pharmacokinetic properties (moderate clearance of 1.2 l/h/kg and a short half‐life of only 1.2 hours).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Contemporary Accounts in Drug Discovery and Development»
Представляем Вашему вниманию похожие книги на «Contemporary Accounts in Drug Discovery and Development» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Contemporary Accounts in Drug Discovery and Development» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












