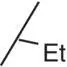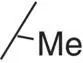Therefore, variations in the carbamate alkoxy group were examined next while leaving the N‐position unsubstituted, with the goal of improving DMPK properties relative to compound 10( Table 3.3).
The introduction of steric bulk in the form of an isopropyl residue (compound 11) led to a strong decrease in potency as well as decreased metabolic stability. Interestingly, potency was slightly increased in cyclobutyl derivative 12; however, the analog was also unfavorable with respect to in vitro clearance. In some cases, the oxetanyl group has improved metabolic stability [38, 39] compared with related alkyl derivatives. Remarkably, the exchange of cyclobutyl for oxetanyl (compound 13) led to a 14‐fold higher stability in rat hepatocytes, but sGC stimulation was weaker than with derivative 10or 12. Since the permeability across Caco‐2 cell monolayers was also very low ( P appA–B = 2 nm/s), combined with a high efflux ratio of 74, compound 13was not pursued further. Additional efforts to improve the overall profile, by the introduction of fluorine or steric bulk (compounds 14and 15), led to a slight increase in potency (MEC = 0.2 μM vs 0.3 μM for 10); however, metabolic stability was in all cases dramatically reduced.
Table 3.2 Properties of the N‐substituted methyl carbamates 1, 3– 9.
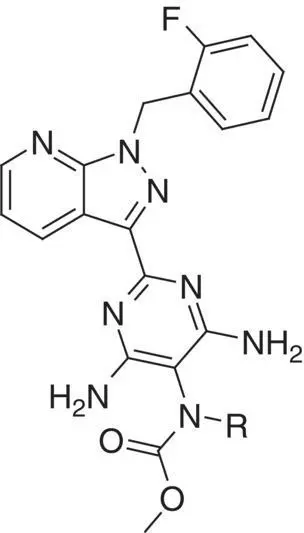 |
| Compound |
R |
cGMP formation MEC a(μM) |
ClogD [37] pH 7.5 |
In vitro clearance (rat hepatocytes) CL b(l/h/kg) |
| 1 |
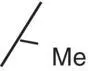 |
0.03 |
1.99 |
0.2 |
| 3 |
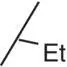 |
0.2 |
2.29 |
0.7 |
| 4 |
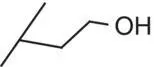 |
0.3 |
1.52 |
3.7 |
| 5 |
 |
0.1 |
2.21 |
0.4 |
| 6 |
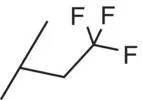 |
0.1 |
2.48 |
0.9 |
| 7 |
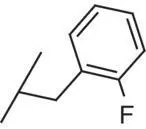 |
0.1 |
3.15 |
3.2 |
| 8 |
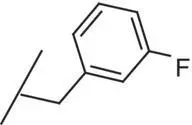 |
0.2 |
3.20 |
3.2 |
| 9 |
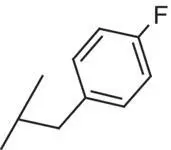 |
0.7 |
3.29 |
2.4 |
aMEC, minimal effective concentration to achieve stimulation of cGMP formation (≥3‐fold increase in basal luminescence) in a recombinant sGC‐overexpressing cell line [36].
Summing up, the carbamate portion of the analogs tolerates various substituents with respect to sGC stimulation but seems to be a crucial region for influencing metabolic stability. All our different strategies, introduction of steric bulk, polarity and substitution by fluorine seemed to offer no promising path forward for optimizing in vitro clearance. Still, the N‐unsubstituted carbamate 10appeared to be optimally constructed in this respect. Thus, the focus of the optimization strategy was shifted away from the carbamate moiety and our next efforts were directed to the central scaffold and the identification of alternative cores that could lead to a potentially superior overall pharmacokinetic profile (modifications summarized in Table 3.4).
Table 3.3 Properties of the N‐H alkyl carbamates 10– 15.
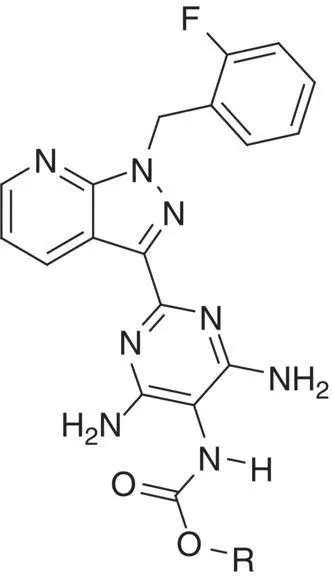 |
| Compound |
R |
cGMP formation MEC a(μM) |
ClogD [37] pH 7.5 |
In vitro clearance (rat hepatocytes) CL b(l/h/kg) |
Caco‐2 P appA–B (nm/s) (Efflux ratio) |
| 10 |
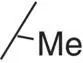 |
0.3 |
1.49 |
0.1 |
79 (5) |
| 11 |
 |
0.8 |
2.10 |
0.9 |
n.d. b |
| 12 |
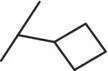 |
0.2 |
2.05 |
1.4 |
23 (25) |
| 13 |
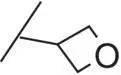 |
2.3 |
1.21 |
0.1 |
2 (74) |
| 14 |
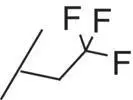 |
0.2 |
2.05 |
2.1 |
n.d. b |
| 15 |
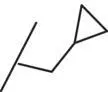 |
0.2 |
2.09 |
1.4 |
n.d. b |
aMEC, minimal effective concentration to achieve stimulation of cGMP formation (≥3‐fold increase in basal luminescence) in a recombinant sGC‐overexpressing cell line [36].
bn.d., not determined.
At first, changes were made to the pyrazolo portion of the molecule, leading to the 1 H ‐pyrazolo[4,3‐ b ]pyridine derivative 16. In addition to the benefit of a shorter synthetic route than that for compound 10, 16proved to be a moderately potent sGC stimulator with an MEC of 1.2 μM and had high metabolic stability when tested in rat hepatocytes. The introduction of substituents at the 6‐position of the 1 H ‐pyrazolo[4,3‐ b ]pyridine core was, in general, not well tolerated leading to a dramatic loss of potency (not shown), with the exception of the 6‐fluoro derivative 17which exhibited an MEC of 0.5 μM and good metabolic stability. To our surprise, compounds 16and 17had very different in vivo clearances when compared in a rat pharmacokinetic experiment (i.v. dosing): fluoro derivative 17had a low clearance of 0.3 l/h/kg versus 1.0 l/h/kg for compound 16. As metabolite identification did not point to metabolism occurring at the pyridine core, the rationale for this threefold reduction in blood clearance remains unclear.
Читать дальше