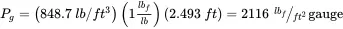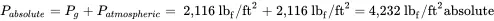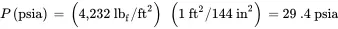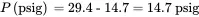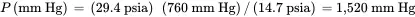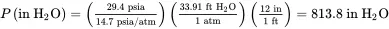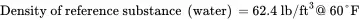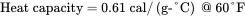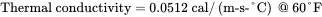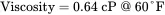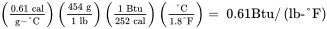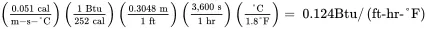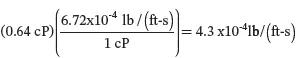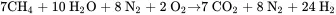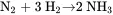Solution . The following key equations are employed:
 (3.5)
(3.5)
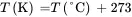 (3.6)
(3.6)
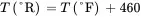 (3.7)
(3.7)
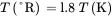 (3.8)
(3.8)
Thus:
Equations 3.5, 3.6, and 3.7 are used as follows:
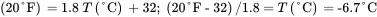
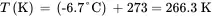
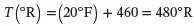
3.9.2 Illustrative Example 2
The height of a liquid column of mercury is 2.493 ft. Assume the density of mercury is 848.7 lb/ft 3and atmospheric pressure is 2116 lb f/ft 2absolute. Calculate the gauge pressure in lb f/ft 2and the absolute pressure in lb f/ft 2, psia, mm Hg, and in H 2O.
Solution . Expressed in various units, the standard atmosphere is equal to:
| 1.0 |
Atmospheres (atm) |
| 33.91 |
Feet of water (ft H 2O) |
| 14.7 |
Pounds force per square inch absolute (psia) |
| 2116 |
Pounds force per square foot absolute (psfa) |
| 29.92 |
Inches of mercury (in Hg) |
| 760.0 |
Millimeters of mercury (mm Hg) |
| 1.013 x 10 5 |
Newtons per square meter (N/m 2) |
The density equation is describing the gauge pressure in terms of the column height and liquid density is:
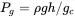 (3.9)
(3.9)
where P g= gauge pressure, ρ = liquid density, g = acceleration of gravity, h = column height, and g c= conversion constant. Thus,
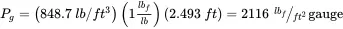
The pressure in lb f/ft 2absolute is:
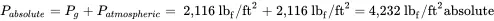
The pressure in psia is;
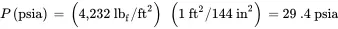
The corresponding gauge pressure in psi is:
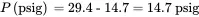
The pressure in mm Hg is:
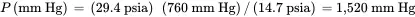
Finally, the pressure in in H 2O is:
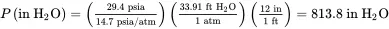
The reader should note that absolute and gauge pressures are usually expressed with units of atm, psi, or mm Hg. This statement also applies to partial pressures. One of the most common units employed to describe pressure drop is inches of H 2O, with the notation in H 2O or IWC (inches of water column).
3.9.3 Illustrative Example 3
Given the following data for liquid methanol, convert the heat capacity, thermal conductivity, and viscosity from SI units to English units:
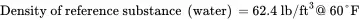
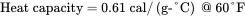
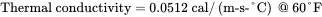
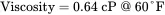
Solution . Convert the heat capacity of methanol from units of cal/(g-°C) to corresponding English units of Btu/ ( lb-°F ) :
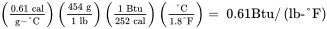
Note that 1.0 Btu/ ( lb-°F ) is equivalent to 1.0 cal/(g-°C). This also applies on a mole basis, i.e. 1 Btu/ ( lbmol-°F ) = 1 cal/ ( gmol-°C ) . The usual notation for heat capacity is Cp . In this book, Cp represents the heat capacity on a mole basis, while cp indicates heat capacity on a mass basis.
Convert the thermal conductivity of methanol from units of cal/(m-s-°C) to corresponding English units of Btu/ ( ft-hr-°F ) :
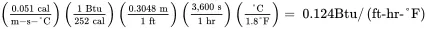
The usual notation for thermal conductivity is k .
C e to lb/(ft-s):
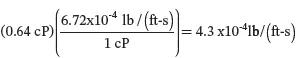
The notation for viscosity is typically µ . The kinematic viscosity, v , is defined by the ratio of viscosity to density, i.e. v = µ / ρ with units of length 2/time. Finally, note that the above physical properties are strong functions of the temperature but weak functions of the pressure. Interestingly, the viscosity of a gas increases with increasing temperature, while the viscosity of a liquid decreases with an increase in temperature. These properties will be revisited in Chapter 04.
3.9.4 Illustrative Example 4
Consider the manufacture of ammonia from natural gas, which can be considered to be pure methane. In the first stage of the process, natural gas is partially oxidized in the presence of steam to produce a synthesis gas containing hydrogen, nitrogen, and carbon dioxide. The carbon dioxide is removed. The remaining mixture of hydrogen and nitrogen is reacted over a catalyst at high pressure to form ammonia. The two reactions for the process, neglecting side reactions and impurities, are as follows:
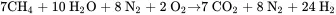
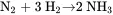
Читать дальше

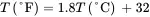 (3.5)
(3.5)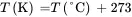 (3.6)
(3.6)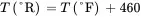 (3.7)
(3.7)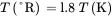 (3.8)
(3.8)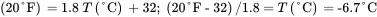
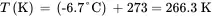
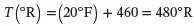
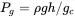 (3.9)
(3.9)