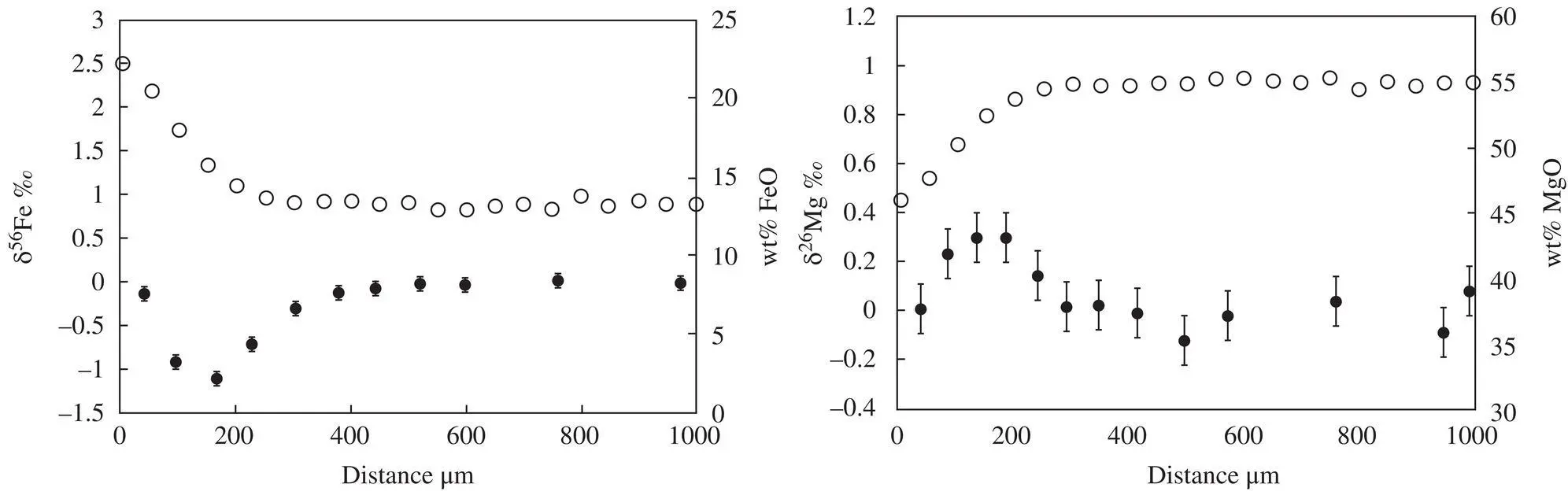
Figure 1.15 Chemical concentration (open circles) and isotopic fractionation (black circles with ±2 sigma error bars when larger than the symbols) measured across one edge of an olivine grain from the Massif Central, France. Note the anti‐correlation of the iron and magnesium isotopic fractionations between 0 and 400 μm.
These data are from Oeser et al. (2015).
The examples described in this section highlight the role of isotopic data as a “fingerprint” of the extent of diffusive mass transport in zoned igneous minerals pyroxene and olivine. The role of the laboratory experiments is to calibrate the “fingerprint,” which can then be used to determine the extent that diffusion is responsible for a given instance of zoning of a natural pyroxene or olivine grain. This isotopic “fingerprint” is especially important when considering whether to use the mineral zoning to determine the thermal history of the host rock.
1.6. ISOTOPE FRACTIONATION BY EVAPORATION FROM SILICATE MELTS
The chemical and isotopic composition of meteorites has had a very prominent role in cosmochemistry in that it provided, among other things, the age of the solar system and the best estimate of the bulk composition of the solar system for all but the most volatile elements. Refractory calcium‐aluminum rich inclusions (CAIs), like the one shown in Fig. 1.16, are particularly important components of primitive chondritic meteorites in that they are the oldest dated materials to have formed in the solar system and, having been present at the creation, they are unique recorders of processes and conditions that prevailed in the early proto‐planetary solar nebula.
A compelling qualitative narrative has been developed regarding the origin and evolution of the Type B CAIs. Type B CAIs, or their precursors, are condensates from a cooling gas of solar composition as evidenced by their being made up of four major minerals – spinel (MgAl 2O 4); melilite (a solid solution between gehlenite, Ca 2Al 2SiO 7, and åkermanite, Ca 2MgSi 2O 7); a Ca‐pyroxene (CaMgSi 2O 6); and anorthite (CaAl 2Si 2O 8) – that are predicted by thermodynamic calculations to be the early condensed minerals from a cooling solar‐composition gas (Grossman 1972). The thermodynamic calculations indicate that the materials that condensed at about 1125°C and became the precursors of the CAIs were solids. The obvious igneous texture of CAIs, like the one shown in Fig. 1.16, is evidence that at some point they must have been melted to a very high degree. In order for the Type B CAIs to have partially melted to the degree required to crystallize large euhedral melilite grains, they must have been reheated to about 1450°C (Stolper, 1982; Stolper & Paque, 1985). A very important characteristic of many CAIs is that they have distinctive oxygen, magnesium, and silicon isotopic compositions. The oxygen isotopic composition of the major minerals in CAIs fall along what appears to be a mixing line between a very 16O‐rich reservoir and a reservoir with oxygen isotopic composition close to that of Earth and other inner solar system materials. The origin of these distinct oxygen reservoirs is still the subject of heated debate and will not be considered here. The range of magnesium and silicon isotopic composition CAIs is more easily understood, in no small part by comparison to the results of laboratory evaporation experiments described in the next section.
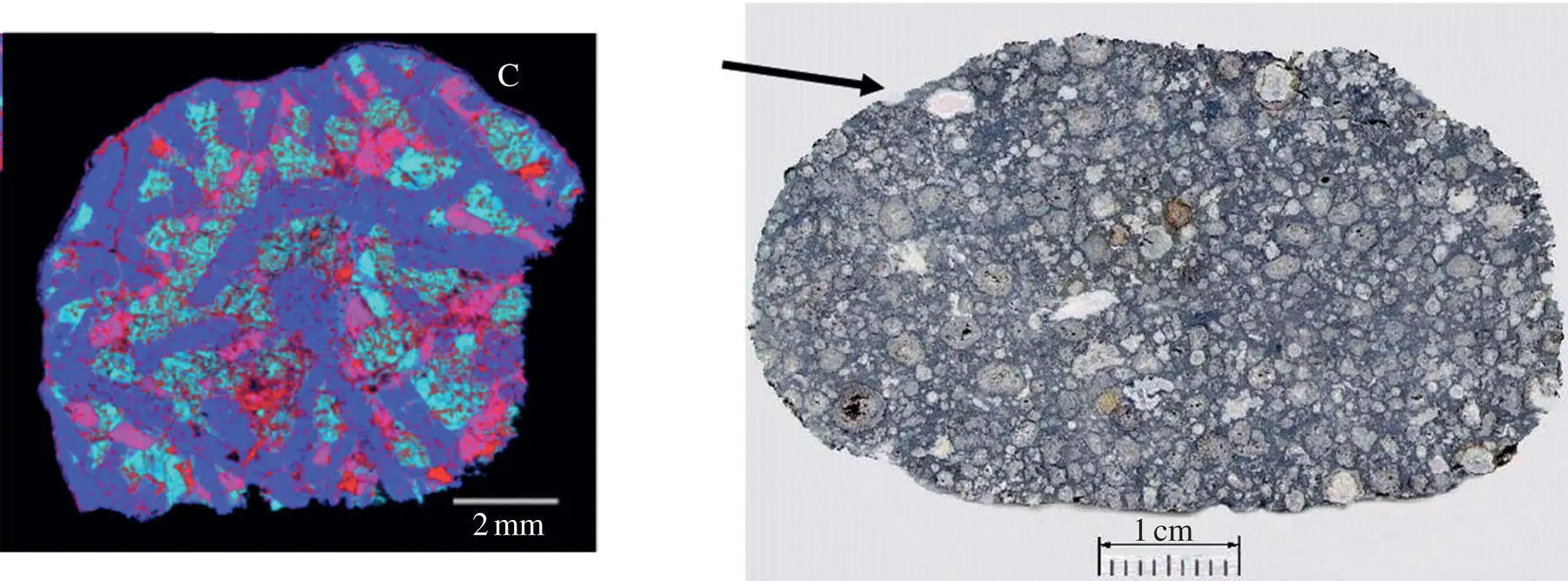
Figure 1.16 The panel on the left shows a false color image of a Type B CAI from the Allende meteorite shown on the right. Type B CAIs are characterized by having large euhedral to sub‐euhedral crystals of melilite shown in blue. The pink grains are anorthite, the light blue grains are pyroxene, and the small red “dots” are spinel. Most of the more or less spherical inclusions in Allende are chondrules while the less abundant, typically larger and more irregular, inclusions are most likely CAIs like the one indicated by the arrow.
1.6.1. The Hertz‐Knudsen Evaporation Equation
The standard formulation for the net flux J iof an element i between a condensed phase and a surrounding gas is the Hertz‐Knudsen equation:
(1.9) 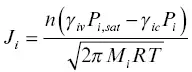
J iis the flux of i in moles per unit area per unit time (positive indicating net evaporation), n is the number of atoms of i in the gas molecule containing i, γ ivis the evaporation coefficient, γ icis the condensation coefficient, P i,satis the saturation vapor pressure of the gas molecule containing i if equilibrium with the condensed phase were realized, P iis the pressure of the gas molecule containing i at the surface of the condensed phase, M iis the molar mass of the gas molecule containing i, R is the gas constant, and T is the absolute temperature. This version of the Hertz‐Knudsen equation applies when there is only one gas molecule containing the element i . In the case of a CAI‐like liquid the dominant gas species of the most volatile elements magnesium and silicon are Mg and SiO (not Si). Since these gas species contain only one atom of the element of interest, n is equal to one in equation 1.9. The total pressure in that part of the solar nebula where the CAIs formed was about 10 –3bars or less (mostly hydrogen with negligible amounts of Mg and SiO) and therefore P i<< P i,sat. Equation 1.9when n =1 and P i<< P i,satbecomes
(1.10) 
The isotopic composition of the evaporation flux is given by the ratio of the flux of isotopes i and j calculated using equation 1.10with the further simplification that because of the thermodynamically ideal behavior of isotopes P i,sat/ P j,sat= N i/ N jwhere N i/ N jis the atom ratio of the isotopes in the evaporating material. The equation for the isotopic composition of the evaporation flux is then
(1.11) 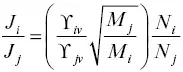
The quantity 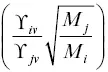 , which we will call α ij, is the kinetic isotope fractionation of the evaporation flux relative to the isotopic ratio of the evaporating material. Prior to having results from vacuum evaporation experiments it was often assumed that
, which we will call α ij, is the kinetic isotope fractionation of the evaporation flux relative to the isotopic ratio of the evaporating material. Prior to having results from vacuum evaporation experiments it was often assumed that 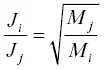 , (i.e., the inverse square root of the mass “law”) but as we will see, this is not correct.
, (i.e., the inverse square root of the mass “law”) but as we will see, this is not correct.
1.6.2. Rayleigh Fractionation
Читать дальше



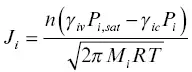

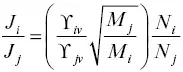
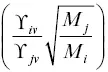 , which we will call α ij, is the kinetic isotope fractionation of the evaporation flux relative to the isotopic ratio of the evaporating material. Prior to having results from vacuum evaporation experiments it was often assumed that
, which we will call α ij, is the kinetic isotope fractionation of the evaporation flux relative to the isotopic ratio of the evaporating material. Prior to having results from vacuum evaporation experiments it was often assumed that 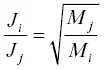 , (i.e., the inverse square root of the mass “law”) but as we will see, this is not correct.
, (i.e., the inverse square root of the mass “law”) but as we will see, this is not correct.










