1 Cover
2 Foreword 1
3 Foreword 2
4 Preface
5 1 Equilibria of Liquid/Vapor Phases
1.1. Exercises 1.2. Problems
1.3. Tests
1.4. Detailed corrections
6 2 Allotropic Solid/Solid Equilibria
2.1. Exercises 2.2. Problems
2.3. Tests
2.4. Detailed corrections
7 3 Solid/Vapor Sublimation Equilibria
3.1. Exercises 3.2. Problems
3.3. Tests
3.4. Detailed corrections
8 4 Process Energetics
4.1. Exercises 4.2. Problems
4.3. Tests
4.4. Detailed corrections
9 Appendix
10 Nomenclature
11 References
12 Index
13 End User License Agreement
1 Chapter 1 Table 1.1. Liquid/liquid solubility limits
Table 1.2. Composition of liquid and vapor phases at equilibrium at 1.009 atm
Table 1.3. Properties of pure bodies (1) and (2)
1 Chapter 1 Figure 1.1. Liquid/vapor equilibrium curve of benzene
Figure 1.2. Curves of g mas a function of the MEK titerFigure 1.3. Curve for 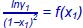 Figure 1.4. Schematic showing the deposition curve as a function of titerFigure 1.5. Curve for y 1= f(x 1) and bubble curveFigure 1.6. Curves for h ex, s exand g exas a function of x 1Figure 1.7. Curve for h m= f(x 1)Figure 1.8. Curve for
Figure 1.4. Schematic showing the deposition curve as a function of titerFigure 1.5. Curve for y 1= f(x 1) and bubble curveFigure 1.6. Curves for h ex, s exand g exas a function of x 1Figure 1.7. Curve for h m= f(x 1)Figure 1.8. Curve for 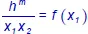 Figure 1.9. Curves for T = f(x 1) and T = f(y 1)Figure 1.10. Curve for α 12= f(x 1)Figure 1.11. T = f (x,y) diagramFigure 1.12. Cyclic transformation at the equilibrium stateFigure 1.13. N 2“pure body” diagram
Figure 1.9. Curves for T = f(x 1) and T = f(y 1)Figure 1.10. Curve for α 12= f(x 1)Figure 1.11. T = f (x,y) diagramFigure 1.12. Cyclic transformation at the equilibrium stateFigure 1.13. N 2“pure body” diagram
2 Chapter 2Figure 2.1. Iron/carbon equilibrium diagramFigure 2.2. Phase diagram of the iron/tin binaryFigure 2.3. Phase diagram of the binary Cu(1)/Pb(2)Figure 2.4. Isobaric l/s equilibrium diagram of Li(1)/Na(2)Figure 2.5. Equilibrium curve of H 2O(1)/H 2O 2(2) mixtureFigure 2.6. Diagram of cadmium/leadFigure 2.7. Diagram of a solubility curve of a componentFigure 2.8. Different phases of the iron/tin diagramFigure 2.9. Deposition curve T=f(x 1)Figure 2.10. Indium/tin phase diagramFigure 2.11. Diagram (T, x 2) at constant P of Li(1)/Na(2)Figure 2.12. Thermal analysis curve (T,t) at constant PFigure 2.13. Phase diagram of water/hydrogen peroxideFigure 2.14. Thermal analysis curve T = f(t)Figure 2.15. Crystallization diagram of the binary HNO 3/H 2OFigure 2.16. Thermal analysis curve of the binary HNO 3/H 2OFigure 2.17. Solid/liquid and liquid/vapor diagrams of the binaryFigure 2.18. Thermal analysis curve of the binary H 2O/C 2H 5OHFigure 2.19. s/l and l/v equilibrium diagrams of the binary H 2O/C 2H 6O 2Figure 2.20. Thermal analysis curve of the 10% glycol mixture
3 Chapter 3Figure 3.1. Curve Cp = f(T)Figure 3.2. Curve lnP = f(1/T)Figure 3.3. Amount of H 2produced as a function of moles of FeFigure 3.4. Equilibrium diagram of a pure bodyFigure 3.5. Breakdown of sublimation processFigure 3.6. Interpolation curve T = f(P)Figure 3.7. Sublimation and evaporation curves of H 2O
4 Chapter 4Figure 4.1. Nozzle 1Figure 4.2. Nozzle 2Figure 4.3. System overview (σ)Figure 4.4. The Lenoir cycleFigure 4.5. Joule cycleFigure 4.6. Dual cycleFigure 4.7. Otto cycleFigure 4.8. Diesel cycleFigure 4.9. Diagram of a heat transformerFigure 4.10. Diagram of dry ice productionFigure 4.11. Diagram of a jetFigure 4.12a. Diagrams of the Carnot cycle in different coordinate systemsFigure 4.12b. Diagrams of the Carnot cycle in different coordinate systemsFigure 4.13. Diagram of the engineFigure 4.14. Cycle of gas in the turbineFigure 4.15. Diagram of a combined systemFigure 4.16. Synoptic diagram of the setupFigure 4.17. Thermodynamic cycle of airFigure 4.18. Curve P = f(V)Figure 4.19. Representation of the cycle in coordinates (T,S)Figure 4.20. Diagram of two different heating modes: (a) cooling using water at ...Figure 4.21. Diagram of the production areaFigure 4.22. Synoptic diagram of the production areaFigure 4.23. Diagram (P, 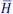 )Figure 4.24. Thermal diagram of the skating rinkFigure 4.25. Joule cycleFigure 4.26. Pulsejet engine cycle
)Figure 4.24. Thermal diagram of the skating rinkFigure 4.25. Joule cycleFigure 4.26. Pulsejet engine cycle
1 Cover
2 Table of Contents Table of Contents 1 Cover 2 Foreword 1 3 Foreword 2 4 Preface 5 1 Equilibria of Liquid/Vapor Phases 1.1. Exercises 1.2. Problems 1.3. Tests 1.4. Detailed corrections 6 2 Allotropic Solid/Solid Equilibria 2.1. Exercises 2.2. Problems 2.3. Tests 2.4. Detailed corrections 7 3 Solid/Vapor Sublimation Equilibria 3.1. Exercises 3.2. Problems 3.3. Tests 3.4. Detailed corrections 8 4 Process Energetics 4.1. Exercises 4.2. Problems 4.3. Tests 4.4. Detailed corrections 9 Appendix 10 Nomenclature 11 References 12 Index 13 End User License Agreement
3 Begin Reading
1 v Table of Contents 1 Cover 2 Foreword 1 3 Foreword 2 4 Preface 5 1 Equilibria of Liquid/Vapor Phases 1.1. Exercises 1.2. Problems 1.3. Tests 1.4. Detailed corrections 6 2 Allotropic Solid/Solid Equilibria 2.1. Exercises 2.2. Problems 2.3. Tests 2.4. Detailed corrections 7 3 Solid/Vapor Sublimation Equilibria 3.1. Exercises 3.2. Problems 3.3. Tests 3.4. Detailed corrections 8 4 Process Energetics 4.1. Exercises 4.2. Problems 4.3. Tests 4.4. Detailed corrections 9 Appendix 10 Nomenclature 11 References 12 Index 13 End User License Agreement
2 ii In memory of my parents
3 iii Series Editor Jean-Claude Charpentier
4 iv First published 2020 in Great Britain and the United States by ISTE Ltd and John Wiley & Sons, Inc. Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the Copyright, Designs and Patents Act 1988, this publication may only be reproduced, stored or transmitted, in any form or by any means, with the prior permission in writing of the publishers, or in the case of reprographic reproduction in accordance with the terms and licenses issued by the CLA. Enquiries concerning reproduction outside these terms should be sent to the publishers at the undermentioned address: ISTE Ltd 27-37 St George’s Road London SW19 4EU UK www.iste.co.uk John Wiley & Sons, Inc. 111 River Street Hoboken, NJ 07030 USA www.wiley.com © ISTE Ltd 2020 The rights of Salah Belaadi to be identified as the author of this work have been asserted by him in accordance with the Copyright, Designs and Patents Act 1988. Library of Congress Control Number: 2019953627 British Library Cataloguing-in-Publication Data A CIP record for this book is available from the British Library ISBN 978-1-78630-514-5
5 vii
6 viii It is clear that studies that discuss thermodynamics of processes must cover chemical thermodynamics (open or closed systems with or without chemical reaction, phase equilibrium) and the energetics of processes (thermal cycles, heat pump, degraded energy, exergy). However, these studies must also be illustrated with examples of real multiscale physicochemical applications. This will prepare or help or contribute to the design, the development and the control of the processes that will be encountered in the factory of the future, by means of methodologies and techniques to obtain reliable thermodynamic data that will contribute to the abundance of data ( Big Data/Industry 4.0 ). A big thank you to Professor Salah Belaadi, leading expert in education and research in the field of thermodynamics of processes, for offering such an instructional and didactic book, whose chapters mainly present exercises oriented towards industrial applications. This book on thermodynamics and energetics of processes is a guide (a vademecum ), which I am personally convinced will be of great benefit to a large number of university teachers and researchers, and engineers and technicians active in today’s economy sector, as well in the very near future. Jean-Claude CHARPENTIER Former director of ENSIC Nancy and ESCPE Lyon, France Former president of the European Federation of Chemical Engineering Laboratoire Réactions et Génie des Procédés CNRS/ENSIC/University of Lorraine
Читать дальше

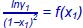 Figure 1.4. Schematic showing the deposition curve as a function of titerFigure 1.5. Curve for y 1= f(x 1) and bubble curveFigure 1.6. Curves for h ex, s exand g exas a function of x 1Figure 1.7. Curve for h m= f(x 1)Figure 1.8. Curve for
Figure 1.4. Schematic showing the deposition curve as a function of titerFigure 1.5. Curve for y 1= f(x 1) and bubble curveFigure 1.6. Curves for h ex, s exand g exas a function of x 1Figure 1.7. Curve for h m= f(x 1)Figure 1.8. Curve for 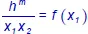 Figure 1.9. Curves for T = f(x 1) and T = f(y 1)Figure 1.10. Curve for α 12= f(x 1)Figure 1.11. T = f (x,y) diagramFigure 1.12. Cyclic transformation at the equilibrium stateFigure 1.13. N 2“pure body” diagram
Figure 1.9. Curves for T = f(x 1) and T = f(y 1)Figure 1.10. Curve for α 12= f(x 1)Figure 1.11. T = f (x,y) diagramFigure 1.12. Cyclic transformation at the equilibrium stateFigure 1.13. N 2“pure body” diagram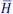 )Figure 4.24. Thermal diagram of the skating rinkFigure 4.25. Joule cycleFigure 4.26. Pulsejet engine cycle
)Figure 4.24. Thermal diagram of the skating rinkFigure 4.25. Joule cycleFigure 4.26. Pulsejet engine cycle










