1 ...8 9 10 12 13 14 ...38 Fig. 1.4shows the 44Ca/ 40Ca fractionation measured by thermal ionization mass spectrometry of purified solutions derived by dissolving thin (~ 0.5 mm) slabs cut perpendicular to the long axis of the recovered glass from experiment RB‐2 run for 15.7 hours at 1450°C and 1.3 GPa, and from a second diffusion couple, RB‐3, run for 12 hours at 1450°C and 1.2 GPa. The calcium isotopic fractionation is reported in the usual per mil notation 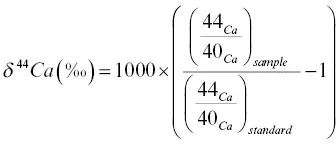 with the 44Ca/ 40Ca of a purified CaCO 3salt used as the standard. The calcium isotopic composition of the rhyolite is within error (± 0.2), the same as that of the basalt, and therefore the initial δ 44Ca across the couple was effectively uniform. The locally negative δ 44Ca in that part of the couple where calcium diffused from the basalt into the rhyolite is evidence of 40Ca having diffused measurably faster than 44Ca.
with the 44Ca/ 40Ca of a purified CaCO 3salt used as the standard. The calcium isotopic composition of the rhyolite is within error (± 0.2), the same as that of the basalt, and therefore the initial δ 44Ca across the couple was effectively uniform. The locally negative δ 44Ca in that part of the couple where calcium diffused from the basalt into the rhyolite is evidence of 40Ca having diffused measurably faster than 44Ca.
The model calculation for the evolution of the major oxide components and the calcium isotopes was formulated using effective binary diffusion coefficients. The conservation equations for total CaO, SiO 2, 40CaO, and 44CaO with effective binary diffusion coefficients for CaO and SiO 2that depend on the local SiO 2content of the melt are
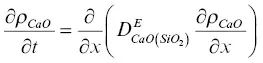
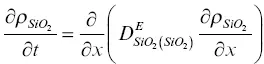
(1.7) 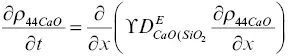
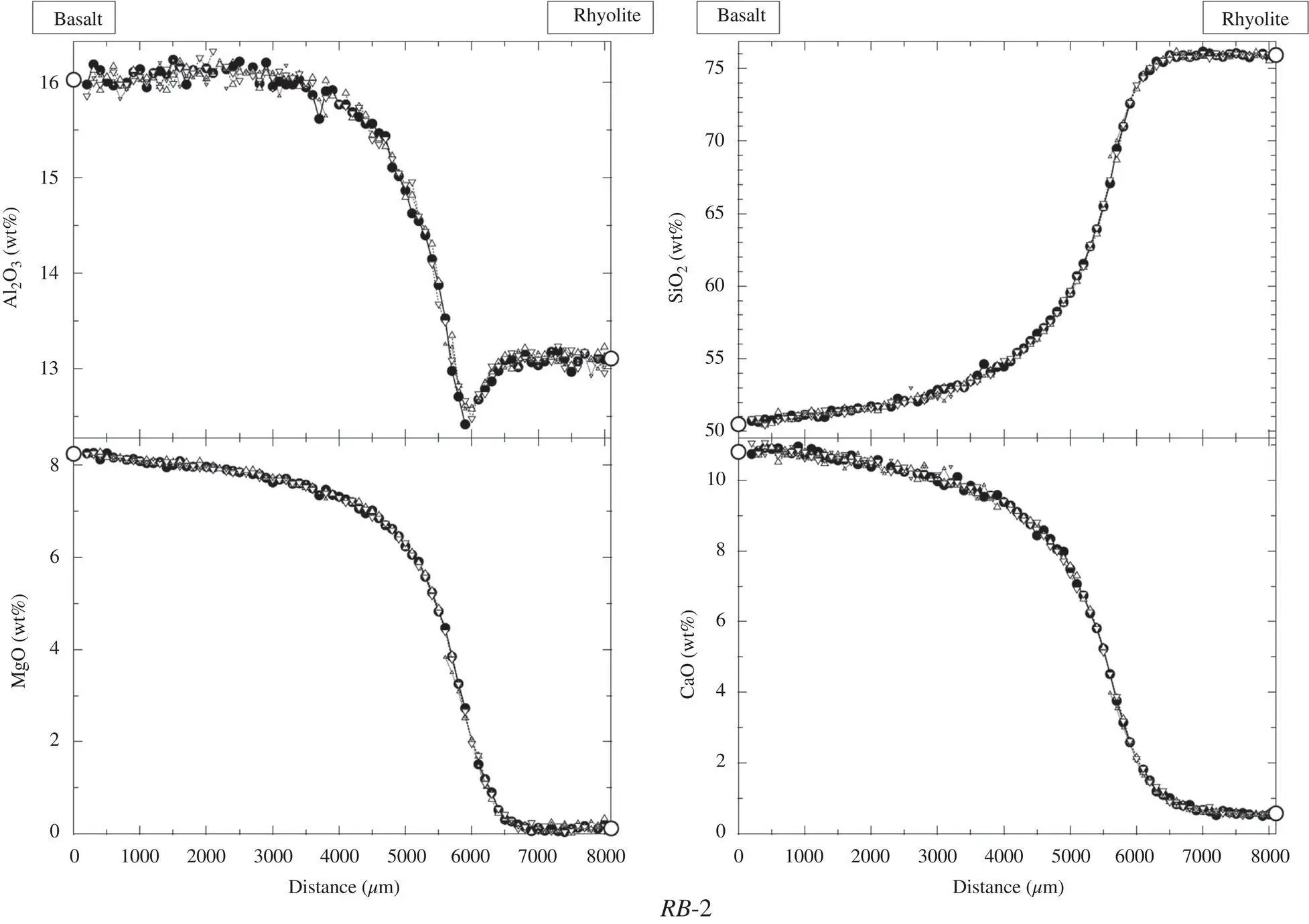
Figure 1.3 The panels show the concentration of major oxides measured along five parallel lines perpendicular to the interface between a natural basalt melt (SUNY MORB) and a natural rhyolite melt (Lake County obsidian) that were juxtaposed and annealed in a piston cylinder assembly. The data from each parallel line is plotted with a different symbol, but because the data measured along the five lines are effectively identical, they are not easily distinguished from each other. The fact that the data measured along the parallel lines are indistinguishable shows that diffusion in this couple was perfectly one‐dimensional.
The data plotted in this figure are from Richter et al. (2003).
The initial condition used for the model result shown in Fig. 1.4was a step function with 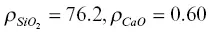 on the rhyolite side and
on the rhyolite side and 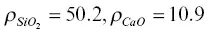 on the basalt side. The units of ρ in equations 1.7are weight percent. The initial 44C/ 40Ca was a constant –0.2‰ across the couple and no‐flux boundary conditions were imposed at both ends. A series of forward‐difference numerical solutions to the conservation equations CaO and SiO 2were calculated until the dependence of the effective diffusion coefficients on the evolving SiO 2content of the melt (i.e.,
on the basalt side. The units of ρ in equations 1.7are weight percent. The initial 44C/ 40Ca was a constant –0.2‰ across the couple and no‐flux boundary conditions were imposed at both ends. A series of forward‐difference numerical solutions to the conservation equations CaO and SiO 2were calculated until the dependence of the effective diffusion coefficients on the evolving SiO 2content of the melt (i.e., 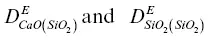 ) was found that simultaneously fit the CaO and SiO 2profiles shown in Fig. 1.3. The isotopic fractionation of calcium was then calculated by repeating the calculation with separate conservation equations for 40Ca and 44Ca. The diffusion coefficient for 40CaO ( 40Ca is ~97 % of natural calcium) was taken to be the same as for the total CaO (i.e.,
) was found that simultaneously fit the CaO and SiO 2profiles shown in Fig. 1.3. The isotopic fractionation of calcium was then calculated by repeating the calculation with separate conservation equations for 40Ca and 44Ca. The diffusion coefficient for 40CaO ( 40Ca is ~97 % of natural calcium) was taken to be the same as for the total CaO (i.e., 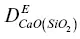 ) while that for 44CaO was reduced by a factor
) while that for 44CaO was reduced by a factor 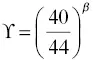 with β determined by fitting the calcium isotopic fractionation data.
with β determined by fitting the calcium isotopic fractionation data.
The solid black line in Fig. 1.4shows the calcium isotopic fractionation calculated using solutions to equations 1.7for 44CaO, and 40CaO, and 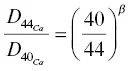 with β = 0.075. The calculated calcium isotope fractionation is a reasonably good fit to the negatively fractionated portion of the data; however, it does not account for the increasingly positive isotope fractionation values towards left end of the couple, which is most noticeable in the RB‐2 data. This isotopic fractionation in the basaltic side of diffusion couple RB‐2 was very surprising, in that it occurs in a part of the couple that had lost very little of its original CaO and thus it would require that some unimaginably large negatively fractionated CaO had to have been removed in order to leave behind the observed positive values. The discrepancy between the isotopic fractionation profile in the basaltic side of couple RB‐2 calculated by Richter and the data measured by DePaolo became a serious point of contention, as each blamed the other for the discrepancy. Several years later it was realized that the positive isotopic fractionations might be due to thermal isotopic fractionation (i.e., Soret diffusion) associated with the typical temperature differences of several tens of degrees centigrade across samples run in piston cylinder assemblies. However, for this to be a viable explanation it would have to be shown that differences of a few tens of degrees across molten basalt can produce calcium isotopic fractionations of several per mil. Soret experiments designed to address this are discussed in Section 1.4.
with β = 0.075. The calculated calcium isotope fractionation is a reasonably good fit to the negatively fractionated portion of the data; however, it does not account for the increasingly positive isotope fractionation values towards left end of the couple, which is most noticeable in the RB‐2 data. This isotopic fractionation in the basaltic side of diffusion couple RB‐2 was very surprising, in that it occurs in a part of the couple that had lost very little of its original CaO and thus it would require that some unimaginably large negatively fractionated CaO had to have been removed in order to leave behind the observed positive values. The discrepancy between the isotopic fractionation profile in the basaltic side of couple RB‐2 calculated by Richter and the data measured by DePaolo became a serious point of contention, as each blamed the other for the discrepancy. Several years later it was realized that the positive isotopic fractionations might be due to thermal isotopic fractionation (i.e., Soret diffusion) associated with the typical temperature differences of several tens of degrees centigrade across samples run in piston cylinder assemblies. However, for this to be a viable explanation it would have to be shown that differences of a few tens of degrees across molten basalt can produce calcium isotopic fractionations of several per mil. Soret experiments designed to address this are discussed in Section 1.4.
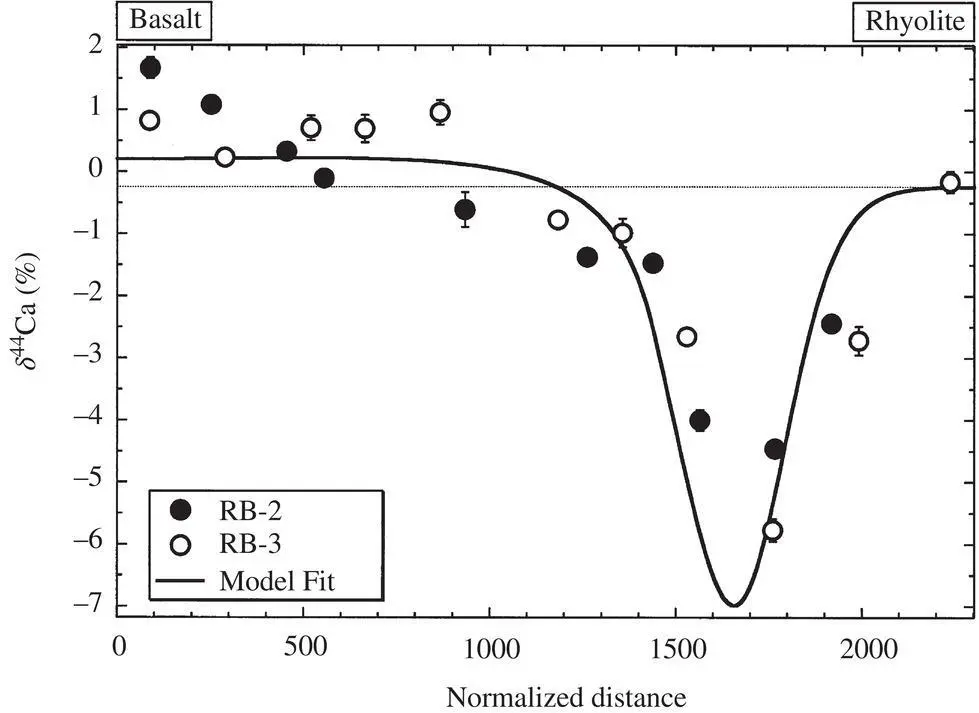
Figure 1.4 Figure taken from Richter et al. (2003) showing the δ 44Ca of slabs cut perpendicular to the long axis of glass recovered from rhyolite‐basalt diffusion couples RB‐2 and RB‐3. A normalized distance scale with units x/t 1/2, where x is in microns and t is the run duration in hours is used in order that the data from the two experiments of different size and duration can be plotted along the same normalized distance axis. The thin horizontal line shows the initial 44C/ 40Ca of the rhyolite and basalt relative that of a CaCO 3salt standard. The thicker black line is the result of a model calculation with the mass‐dependence of the diffusion coefficient of calcium calculated as 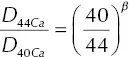 with β = 0.075.
with β = 0.075.
1.3.2. Isotope Fractionation between Melts from a Natural Setting
The relevance to geology of the kinetic isotope fractionations by diffusion in melts as documented by the laboratory experiments depends on whether similar fractionations can be found in natural settings. Chopra et al. (2012) addressed this in a detailed study, in which the magnesium isotopic fractionations in diffusion couples juxtaposing felsic and mafic powdered rock samples from the Vinalhaven intrusive complex in Maine were compared with the isotopic fractionations they measured across an exposed contact between these two rock types. Fig. 1.5shows the piston cylinder assembly used by Chopra et al. (2012) for the experiments along with a backscattered electron image of glass recovered from their experiment GBM‐2. The figure also shows the temperatures measured in the piston cylinder assembly above and below the sample and a temperature profile extrapolated the measured temperatures into the sample while it was at run conditions. The temperatures in the piston cylinder assembly were determined by the thickness of spinel that grew where Al 2O 3and MgO were in contact at various places in the assembly based on a calibration of the growth rate of spinel as a function of temperature and pressure by Watson et al. (2002). The calibration is used to convert the spinel thickness into the time‐averaged temperature over the duration of the experiment.
Читать дальше

 with the 44Ca/ 40Ca of a purified CaCO 3salt used as the standard. The calcium isotopic composition of the rhyolite is within error (± 0.2), the same as that of the basalt, and therefore the initial δ 44Ca across the couple was effectively uniform. The locally negative δ 44Ca in that part of the couple where calcium diffused from the basalt into the rhyolite is evidence of 40Ca having diffused measurably faster than 44Ca.
with the 44Ca/ 40Ca of a purified CaCO 3salt used as the standard. The calcium isotopic composition of the rhyolite is within error (± 0.2), the same as that of the basalt, and therefore the initial δ 44Ca across the couple was effectively uniform. The locally negative δ 44Ca in that part of the couple where calcium diffused from the basalt into the rhyolite is evidence of 40Ca having diffused measurably faster than 44Ca.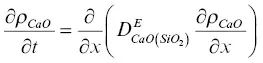
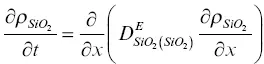
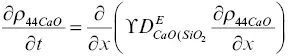

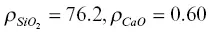 on the rhyolite side and
on the rhyolite side and 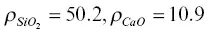 on the basalt side. The units of ρ in equations 1.7are weight percent. The initial 44C/ 40Ca was a constant –0.2‰ across the couple and no‐flux boundary conditions were imposed at both ends. A series of forward‐difference numerical solutions to the conservation equations CaO and SiO 2were calculated until the dependence of the effective diffusion coefficients on the evolving SiO 2content of the melt (i.e.,
on the basalt side. The units of ρ in equations 1.7are weight percent. The initial 44C/ 40Ca was a constant –0.2‰ across the couple and no‐flux boundary conditions were imposed at both ends. A series of forward‐difference numerical solutions to the conservation equations CaO and SiO 2were calculated until the dependence of the effective diffusion coefficients on the evolving SiO 2content of the melt (i.e., 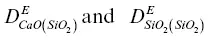 ) was found that simultaneously fit the CaO and SiO 2profiles shown in Fig. 1.3. The isotopic fractionation of calcium was then calculated by repeating the calculation with separate conservation equations for 40Ca and 44Ca. The diffusion coefficient for 40CaO ( 40Ca is ~97 % of natural calcium) was taken to be the same as for the total CaO (i.e.,
) was found that simultaneously fit the CaO and SiO 2profiles shown in Fig. 1.3. The isotopic fractionation of calcium was then calculated by repeating the calculation with separate conservation equations for 40Ca and 44Ca. The diffusion coefficient for 40CaO ( 40Ca is ~97 % of natural calcium) was taken to be the same as for the total CaO (i.e., 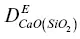 ) while that for 44CaO was reduced by a factor
) while that for 44CaO was reduced by a factor 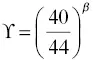 with β determined by fitting the calcium isotopic fractionation data.
with β determined by fitting the calcium isotopic fractionation data.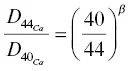 with β = 0.075. The calculated calcium isotope fractionation is a reasonably good fit to the negatively fractionated portion of the data; however, it does not account for the increasingly positive isotope fractionation values towards left end of the couple, which is most noticeable in the RB‐2 data. This isotopic fractionation in the basaltic side of diffusion couple RB‐2 was very surprising, in that it occurs in a part of the couple that had lost very little of its original CaO and thus it would require that some unimaginably large negatively fractionated CaO had to have been removed in order to leave behind the observed positive values. The discrepancy between the isotopic fractionation profile in the basaltic side of couple RB‐2 calculated by Richter and the data measured by DePaolo became a serious point of contention, as each blamed the other for the discrepancy. Several years later it was realized that the positive isotopic fractionations might be due to thermal isotopic fractionation (i.e., Soret diffusion) associated with the typical temperature differences of several tens of degrees centigrade across samples run in piston cylinder assemblies. However, for this to be a viable explanation it would have to be shown that differences of a few tens of degrees across molten basalt can produce calcium isotopic fractionations of several per mil. Soret experiments designed to address this are discussed in Section 1.4.
with β = 0.075. The calculated calcium isotope fractionation is a reasonably good fit to the negatively fractionated portion of the data; however, it does not account for the increasingly positive isotope fractionation values towards left end of the couple, which is most noticeable in the RB‐2 data. This isotopic fractionation in the basaltic side of diffusion couple RB‐2 was very surprising, in that it occurs in a part of the couple that had lost very little of its original CaO and thus it would require that some unimaginably large negatively fractionated CaO had to have been removed in order to leave behind the observed positive values. The discrepancy between the isotopic fractionation profile in the basaltic side of couple RB‐2 calculated by Richter and the data measured by DePaolo became a serious point of contention, as each blamed the other for the discrepancy. Several years later it was realized that the positive isotopic fractionations might be due to thermal isotopic fractionation (i.e., Soret diffusion) associated with the typical temperature differences of several tens of degrees centigrade across samples run in piston cylinder assemblies. However, for this to be a viable explanation it would have to be shown that differences of a few tens of degrees across molten basalt can produce calcium isotopic fractionations of several per mil. Soret experiments designed to address this are discussed in Section 1.4.
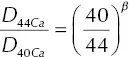 with β = 0.075.
with β = 0.075.










