1 ...7 8 9 11 12 13 ...38
1.2.3. Self‐Diffusion Coefficients
A very basic type of diffusion coefficient is the self‐diffusion coefficient that measures of the intrinsic mobility of a species. A common experimental approach for measuring self‐diffusion coefficients is to locally alter the isotopic composition of the element of interest in an otherwise homogeneous medium and observe the rate at which the isotopic difference spreads. In effect, this eliminates all diffusive coupling to the other species because their gradients are all zero. The self‐diffusion coefficient 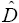 of elements in silicate liquids can be very different from each other. For example, Liang et al. (1996a) reported a large set of experimentally determined self‐diffusion coefficients as function of composition in molten CaO‐Al 2O 3‐SiO 2showing that
of elements in silicate liquids can be very different from each other. For example, Liang et al. (1996a) reported a large set of experimentally determined self‐diffusion coefficients as function of composition in molten CaO‐Al 2O 3‐SiO 2showing that 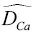 ~ 10×
~ 10× 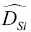 ,
, 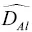 ~2×
~2× 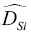 , and
, and 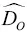 ~1 to 2×
~1 to 2× 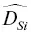 . These differences in self‐diffusion are in marked contrast to the very similar magnitude of the effective binary diffusion coefficients in a molten rhyolite‐basalt diffusion couple. Fig. 1.1shows this by overlaying diffusion profiles measured by Richter et al. (2003) in a diffusion couple in which natural rhyolite liquid and a natural basaltic liquid were juxtaposed and annealed in a piston cylinder experiment. The remarkably similar diffusive behavior the major oxides shown in Fig. 1.1is the result of the fluxes of MgO, CaO, FeO, and K 2O all being strongly coupled to the concentration gradient of SiO 2via the off‐diagonal terms of the diffusion matrix. There are two reasons for this strong coupling with SiO 2. One reason is that the sum of volume fluxes of all the components must add up to zero and thus the fluxes are rate limited by the large and sluggish volume flux of SiO 2that has to balance the fluxes of the other components. The second reason for the coupling is that the chemical potential gradients of the major oxides, which drive their diffusion, depend on the local SiO 2content of the melt (see Liang et al., 1967 for a detailed discussion of the causes of diffusive coupling in silicate liquids).
. These differences in self‐diffusion are in marked contrast to the very similar magnitude of the effective binary diffusion coefficients in a molten rhyolite‐basalt diffusion couple. Fig. 1.1shows this by overlaying diffusion profiles measured by Richter et al. (2003) in a diffusion couple in which natural rhyolite liquid and a natural basaltic liquid were juxtaposed and annealed in a piston cylinder experiment. The remarkably similar diffusive behavior the major oxides shown in Fig. 1.1is the result of the fluxes of MgO, CaO, FeO, and K 2O all being strongly coupled to the concentration gradient of SiO 2via the off‐diagonal terms of the diffusion matrix. There are two reasons for this strong coupling with SiO 2. One reason is that the sum of volume fluxes of all the components must add up to zero and thus the fluxes are rate limited by the large and sluggish volume flux of SiO 2that has to balance the fluxes of the other components. The second reason for the coupling is that the chemical potential gradients of the major oxides, which drive their diffusion, depend on the local SiO 2content of the melt (see Liang et al., 1967 for a detailed discussion of the causes of diffusive coupling in silicate liquids).
1.2.4. Thermal (Soret) Diffusion Coefficients
The flux equation of a system that is inhomogeneous in both composition and temperature will have terms proportional to both the chemical gradient and the temperature gradient. Expanding the binary diffusion representation of the flux to include the effect of a temperature gradient results in a binary flux equation of the form (Tyrell, 1961)
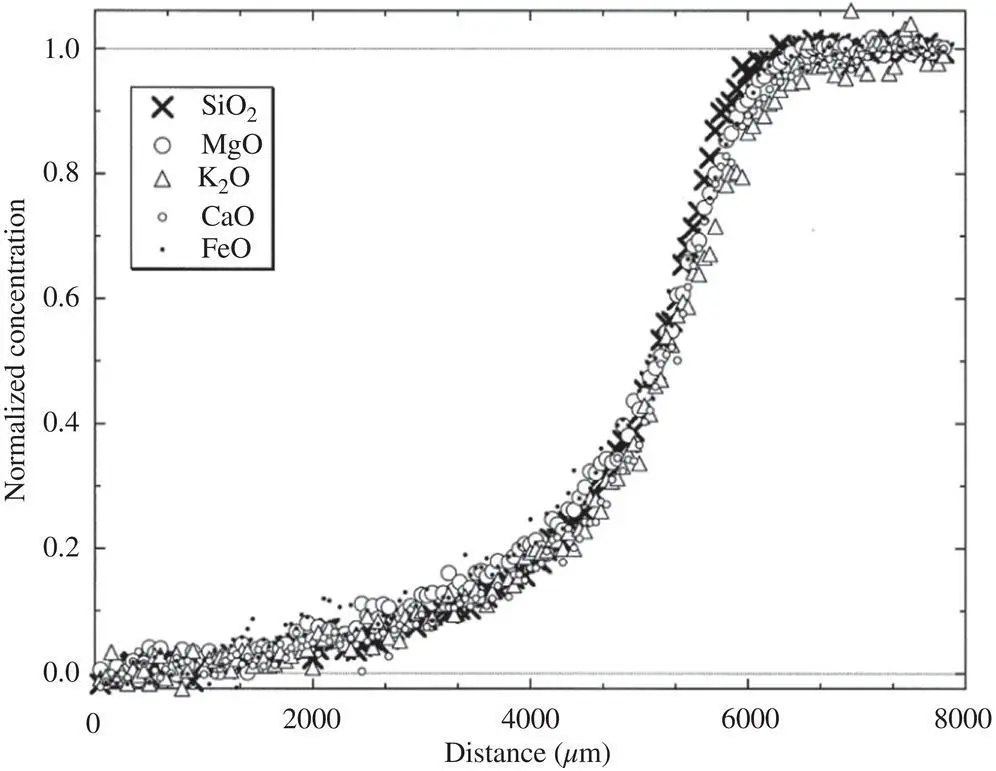
Figure 1.1 Normalized concentration of CaO, MgO, K2O, FeO, and SiO2 measured across glass recovered from a molten rhyolite‐basalt diffusion experiment are superimposed to show the similarity of the effective diffusivity of all the major oxide components. For the purpose of this comparison, the concentration differences were normalized by (C‐CL)/(CH‐CL), where C is the concentration of a component along the profile, CL is its concentration in the low end‐member, and CH is its concentration in high end‐member.
Figure taken from Richter et al. (2003).
(1.6) 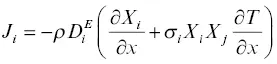
where ρ is the bulk density, 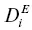 is the effective binary diffusion coefficient of i in a mixture of components i and j , X iand X jare the mass fractions of i and j , and σ iis the Soret coefficient. For present purposes, the term “Soret diffusion” will be used when the mass transport in a silicate liquid is due to fluxes driven by both chemical and temperature differences.
is the effective binary diffusion coefficient of i in a mixture of components i and j , X iand X jare the mass fractions of i and j , and σ iis the Soret coefficient. For present purposes, the term “Soret diffusion” will be used when the mass transport in a silicate liquid is due to fluxes driven by both chemical and temperature differences.
1.3. KINETIC ISOTOPE FRACTIONATION DURING DIFFUSION BETWEEN NATURAL MELTS
The earliest report of measurable differences in the mobility of isotopes of a major element in a silicate liquid was reported by Richter et al. (1999) based on the results of a diffusion experiment involving an isothermal and isochemical CaO‐Al 2O 3‐SiO 2melt with an initial step with different concentration of 48Ca and 40Ca but with the same isotopic ratio of 48Ca/ 40Ca on both sides of the step. When annealed, this setup would cause 48Ca and 40Ca to race each other as they diffused into the low concentration side of the couple. A well‐resolved isotopic fractionation in the glass recovered from this experiment showed that 40Ca had diffused measurably faster than 48Ca. The relative mobility of the calcium isotopes was reported as D 48 /D 40= (40/48) βwith β = 0.075 ± 0.025. Subsequent laboratory experiments by Richter et al. (2003; 2008; 2009b; 2014a) addressed the question of whether the relative mobility of isotopes seen in isochemical experiments would also arise in molten systems where chemical potential gradients are present and where there can be significant coupling between the various diffusing components of the melt.
1.3.1. Laboratory Experiments Documenting Ca Isotope Fractionation by Diffusion Between Molten Rhyolite and Basalt
The type of piston cylinder assembly used by Richter et al. (2003) to determine the isotopic fractionation of calcium and lithium in rhyolite‐basalt diffusion couples is shown schematically in Fig. 1.2.
Fig. 1.3shows the weight percent of major oxide components measured along five parallel lines along the long dimension of the glass recovered from experiment RB‐2. The data from all five lines fall along common smooth curves that are not symmetric with respect to the original interface between the rhyolite and basalt because the rate of diffusion is much faster in the lower SiO 2content of the basalt side of the couple. The Al 2O 3profile is a classic example of uphill diffusion in that the low concentration side of the couple became lower than the initial concentration. This uphill diffusion is an indication that the flux of Al 2O 3is dominated by off‐diagonal terms in the diffusion matrix. Except for Al 2O 3, an effective binary diffusion coefficient D Ecan be derived from fits to the concentration data of all the other major oxides. Experiment RB‐2 was one of two that were run to determine whether calcium isotopes would become measurably fractionated as calcium diffused from the basalt into the rhyolite.
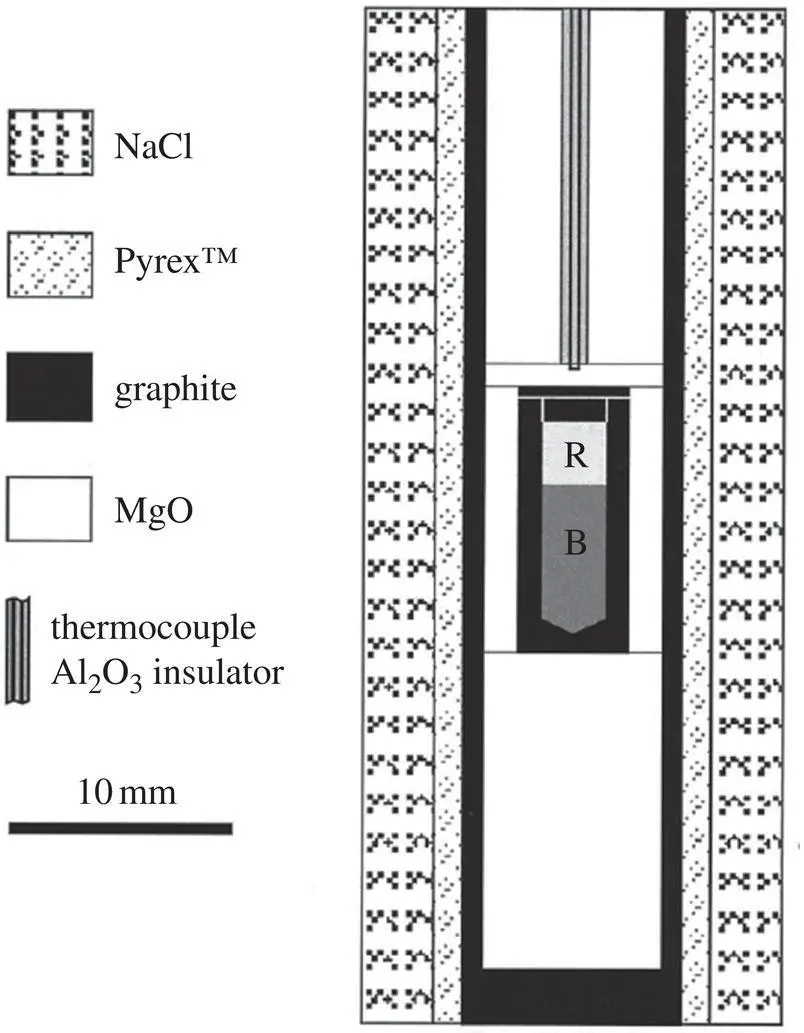
Figure 1.2 A schematic of the piston cylinder assembly used by Richter et al. (2003) to study isotope fractionation by diffusion between molten rhyolite and basalt.
Читать дальше

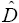 of elements in silicate liquids can be very different from each other. For example, Liang et al. (1996a) reported a large set of experimentally determined self‐diffusion coefficients as function of composition in molten CaO‐Al 2O 3‐SiO 2showing that
of elements in silicate liquids can be very different from each other. For example, Liang et al. (1996a) reported a large set of experimentally determined self‐diffusion coefficients as function of composition in molten CaO‐Al 2O 3‐SiO 2showing that 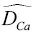 ~ 10×
~ 10× 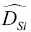 ,
, 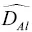 ~2×
~2× 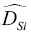 , and
, and 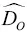 ~1 to 2×
~1 to 2× 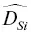 . These differences in self‐diffusion are in marked contrast to the very similar magnitude of the effective binary diffusion coefficients in a molten rhyolite‐basalt diffusion couple. Fig. 1.1shows this by overlaying diffusion profiles measured by Richter et al. (2003) in a diffusion couple in which natural rhyolite liquid and a natural basaltic liquid were juxtaposed and annealed in a piston cylinder experiment. The remarkably similar diffusive behavior the major oxides shown in Fig. 1.1is the result of the fluxes of MgO, CaO, FeO, and K 2O all being strongly coupled to the concentration gradient of SiO 2via the off‐diagonal terms of the diffusion matrix. There are two reasons for this strong coupling with SiO 2. One reason is that the sum of volume fluxes of all the components must add up to zero and thus the fluxes are rate limited by the large and sluggish volume flux of SiO 2that has to balance the fluxes of the other components. The second reason for the coupling is that the chemical potential gradients of the major oxides, which drive their diffusion, depend on the local SiO 2content of the melt (see Liang et al., 1967 for a detailed discussion of the causes of diffusive coupling in silicate liquids).
. These differences in self‐diffusion are in marked contrast to the very similar magnitude of the effective binary diffusion coefficients in a molten rhyolite‐basalt diffusion couple. Fig. 1.1shows this by overlaying diffusion profiles measured by Richter et al. (2003) in a diffusion couple in which natural rhyolite liquid and a natural basaltic liquid were juxtaposed and annealed in a piston cylinder experiment. The remarkably similar diffusive behavior the major oxides shown in Fig. 1.1is the result of the fluxes of MgO, CaO, FeO, and K 2O all being strongly coupled to the concentration gradient of SiO 2via the off‐diagonal terms of the diffusion matrix. There are two reasons for this strong coupling with SiO 2. One reason is that the sum of volume fluxes of all the components must add up to zero and thus the fluxes are rate limited by the large and sluggish volume flux of SiO 2that has to balance the fluxes of the other components. The second reason for the coupling is that the chemical potential gradients of the major oxides, which drive their diffusion, depend on the local SiO 2content of the melt (see Liang et al., 1967 for a detailed discussion of the causes of diffusive coupling in silicate liquids).
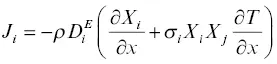
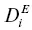 is the effective binary diffusion coefficient of i in a mixture of components i and j , X iand X jare the mass fractions of i and j , and σ iis the Soret coefficient. For present purposes, the term “Soret diffusion” will be used when the mass transport in a silicate liquid is due to fluxes driven by both chemical and temperature differences.
is the effective binary diffusion coefficient of i in a mixture of components i and j , X iand X jare the mass fractions of i and j , and σ iis the Soret coefficient. For present purposes, the term “Soret diffusion” will be used when the mass transport in a silicate liquid is due to fluxes driven by both chemical and temperature differences.











