Richard J. Rossi - Applied Biostatistics for the Health Sciences
Здесь есть возможность читать онлайн «Richard J. Rossi - Applied Biostatistics for the Health Sciences» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Applied Biostatistics for the Health Sciences
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Applied Biostatistics for the Health Sciences: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Applied Biostatistics for the Health Sciences»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
APPLIED BIOSTATISTICS FOR THE HEALTH SCIENCES Applied Biostatistics for the Health Sciences
Applied Biostatistics for the Health Sciences
Applied Biostatistics for the Health Sciences — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Applied Biostatistics for the Health Sciences», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
1.45 In the clinical trial Herbal Medicine ‘Eungyosan’ and ‘Samsoeum’ for Common Cold (NCT04073511) which is summarized at https://clinicaltrials.gov/ct2/show/nct04073511, determine theestimated enrollment in this experiment.treatments in this experiment.country where this clinical trial will take place.age range of the participants in this experiment.
1.46 In the clinical trial Topical Calcipotriene Treatment for Breast Cancer Immunoprevention (NCT03596073) which is summarized at https://clinicaltrials.gov/ct2/show/nct03596073, determine thetreatments in this experiment.locations where this clinical trial will take place.estimated enrollment in this experiment.start date of this study.estimated study completion date.
1.47 In the clinical trial Melanoma Biomarker Study (NCT00348088) which is summarized at https://clinicaltrials.gov/ct2/show/nct00348088, determinethe actual enrollment in this observational study.whether this is a prospective or retrospective study.the location of this study.
1.48 In the clinical trial Validation of PSFS in Carpal Tunnel Syndrome (NCT03909373) which is summarized at https://clinicaltrials.gov/ct2/show/nct03909373, determinethe estimated enrollment in this observational study.whether this is a prospective or retrospective study.the inclusion criteria for participants in this study.the location of this study.
1.49 For the clinical trial NCT04372602 summarized at https://clinicaltrials.gov/ct2/show/NCT04372602, determinethe disease/condition being studied.whether the trial is an experiment or an observational study.
1.50 For the clinical trial NCT03278119 summarized at https://clinicaltrials.gov/crt2/show/nct03278119, determinethe disease/condition being studied.whether the trial is an experiment or an observational study.
1.51 For the clinical trial NCT01370538 summarized at https://clinicaltrials.gov/ct2/show/NCT01370538, determinethe disease/condition being studied.whether the trial is an experiment or an observational study.
1.52 For the clinical trial NCT04591834 summarized at https://clinicaltrials.gov/ct2/show/NCT04591834, determinethe disease/condition being studied.whether the trial is an experiment or an observational study.
CHAPTER 2 DESCRIBING POPULATIONS
IN THEplanning stages of a research project, a set of research questions is developed and refined. Once a well-defined set of research questions has been developed, a target population will be identified so that the goals of the research project can be attained by sampling the target population. The target population is the reference population about which the research questions apply, from which the sample data will be collected, and is the population that statistical inferences will be made about. The research questions will also define the set of variables that must be measured on each unit that is sampled. A variable is a characteristic that will be measured on the units of the target population.
It is important to note that each variable will then have its own population of values. That is, because the units of the population will differ to some degree, a variable will often reflect the differences between the population units and take on several different values. The research questions will also need to be converted into questions about the particular characteristics of the population of values of a variable. In particular, the research questions must be expressed as questions concerning the parameters of the population of values of the variable so that statistical methods can be used to estimate, test, or predict the values of the parameters of interest.
In converting the research questions to questions about the parameters of a population, it is critical for a biomedical researcher and a biostatistician to work together to identify the parameters of the population that are relevant to the research questions being asked. The biomedical research team will also need to determine all of the variables that will be measured before collecting the sample data. In a well-designed research project, it is likely that a biomedical researcher and a biostatistician will work together to design the appropriate sampling plan and to determine the appropriate statistical methodology that will provide meaningful and accurate statistical inferences about the target population and the research questions.
2.1 Populations and Variables
In a properly designed biomedical research study, a well-defined target population and a particular set of research questions dictate the variables that should be measured on the units being studied in the research project. In most research problems, there are many variables that must be measured on each unit in the population. The outcome variables that are of primary interest are called the response variables , and the variables that are believed to explain the response variables are called the explanatory variables or predictor variables . For example, in a clinical trial designed to study the efficacy of a specialized treatment designed to reduce the size of a malignant tumor, the following explanatory variables might be recorded for each patient in the study: age, gender, race, weight, height, blood type, blood pressure, and oxygen uptake. The response variable in this study might be change in the size of the tumor.
Variables come in a variety of different types; however, each variable can be classified as being either quantitative or qualitative in nature. A variable that takes on only numeric values is a quantitative variable , and a variable that takes on non-numeric values is called a qualitative variable or a categorical variable . Note that a variable is a quantitative or qualitative variable based on the possible values the variable can take on.
Example 2.1
In a study of obesity in the population of children aged 10 or less in the United States, some possible quantitative variables that might be measured include age, height, weight, heart rate, body mass index, and percent body fat; some qualitative variables that might be measured on this population include gender, eye color, race, and blood type. A likely choice for the response variable in this study would be the qualitative variable Obese defined by
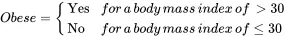
2.1.1 Qualitative Variables
Qualitative variables take on nonnumeric values and are usually used to represent a distinct quality of a population unit. When the possible values of a qualitative variable have no intrinsic ordering, the variable is called a nominal variable ; when there is a natural ordering of the possible values of the variable, then the variable is called an ordinal variable . An example of a nominal variable is Blood Type where the standard values for blood type are A, B, AB, and O. Clearly, there is no intrinsic ordering of these blood types, and hence, Blood Type is a nominal variable. An example of an ordinal variable is the variable Pain where a subject is asked to describe their pain verbally as
No pain,
Mild pain,
Discomforting pain,
Distressing pain,
Intense pain,
Excruciating pain.
In this case, since the verbal descriptions describe increasing levels of pain, there is a clear ordering of the possible values of the variable Pain levels, and therefore, Pain is an ordinal qualitative variable.
Example 2.2
In the Framingham Heart Study of coronary heart disease, the following two nominal qualitative variables were recorded:
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Applied Biostatistics for the Health Sciences»
Представляем Вашему вниманию похожие книги на «Applied Biostatistics for the Health Sciences» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Applied Biostatistics for the Health Sciences» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.