Bohr held up his decision between Cambridge and Manchester until he could go over everything with Harald, who visited him in Cambridge in January 1912 for the purpose. Then Bohr eagerly wrote Rutherford for permission to study at Manchester, as they had discussed in December. Rutherford had advised him then not to give up on Cambridge too quickly—Manchester is always here, he told him, it won’t run away—and so Bohr proposed to arrive for spring term, which began in late March. 266Rutherford gladly agreed. Bohr felt he was being wasted at Cambridge. He wanted substantial work.
His first six weeks in Manchester he spent following “an introductory course on the experimental methods of radioactive research,” with Geiger and Marsden among the instructors. 267He continued pursuing his independent studies in electron theory. He began a lifelong friendship with a young Hungarian aristocrat, George de Hevesy, a radiochemist with a long, sensitive face dominated by a towering nose. De Hevesy’s father was a court councillor, his mother a baroness; as a child he had hunted partridge in the private game park of the Austro-Hungarian emperor Franz Josef next to his grandfather’s estate. Now he was working to meet a challenge Rutherford had thrown at him one day to separate radioactive decay products from their parent substances. Out of that work he developed over the next several decades the science of using radioactive tracers in medical and biological research, one more useful offspring of Rutherford’s casual but fecund paternity.
Bohr learned about radiochemistry from de Hevesy. 268He began to see connections with his electron-theory work. His sudden burst of intuitions then was spectacular. He realized in the space of a few weeks that radioactive properties originated in the atomic nucleus but chemical properties depended primarily on the number and distribution of electrons. He realized—the idea was wild but happened to be true—that since the electrons determined the chemistry and the total positive charge of the nucleus determined the number of electrons, an element’s position on the periodic table of the elements was exactly the nuclear charge (or “atomic number”): hydrogen first with a nuclear charge of 1, then helium with a nuclear charge of 2 and so on up to uranium at 92.
De Hevesy remarked to him that the number of known radio elements already far outnumbered the available spaces on the periodic table and Bohr made more intuitive connections. Soddy had pointed out that the radio elements were generally not new elements, only variant physical forms of the natural elements (he would soon give them their modern name, isotopes). Bohr realized that the radio elements must have the same atomic number as the natural elements with which they were chemically identical. That enabled him to rough out what came to be called the radioactive displacement law: that when an element transmutes itself through radioactive decay it shifts its position on the periodic table two places to the left if it emits an alpha particle (a helium nucleus, atomic number 2), one place to the right if it emits a beta ray (an energetic electron, which leaves behind in the nucleus an extra positive charge).
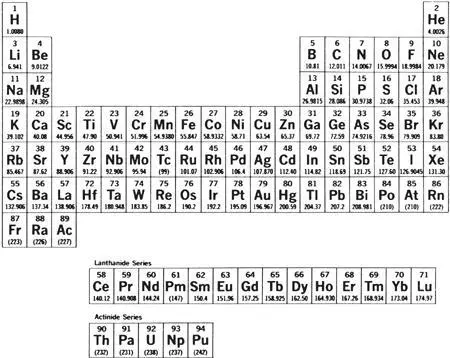
Periodic table of the elements. The lanthanide series (“rare earths”), beginning with lanthanum (57), and the actinide series, which begins with actinium (89) and includes thorium (90) and uranium (92), are chemically similar. Other families of elements read vertically down the table—at the far right, for example, the noble gases: helium, neon, argon, krypton, xenon, radon.
All these first rough insights would be the work of other men’s years to anchor soundly in theory and experiment. Bohr ran them in to Rutherford. To his surprise, he found the discoverer of the nucleus cautious about his own discovery. “Rutherford… thought that the meagre evidence [so far obtained] about the nuclear atom was not certain enough to draw such consequences,” Bohr recalled. 269“And I said to him that I was sure that it would be the final proof of his atom.” If not convinced, Rutherford was at least impressed; when de Hevesy asked him a question about radiation one day Rutherford responded cheerfully, “Ask Bohr!” 270
Rutherford was well prepared for surprises, then, when Bohr came to see him again in mid-June. Bohr told Harald what he was on to in a letter on June 19, after the meeting:
It could be that I’ve perhaps found out a little bit about the structure of atoms. You must not tell anyone anything about it, otherwise I certainly could not write you this soon. If I’m right, it would not be an indication of the nature of a possibility… but perhaps a little piece of reality…. You understand that I may yet be wrong, for it hasn’t been worked out fully yet (but I don’t think so); nor do I believe that Rutherford thinks it’s completely wild; he is the right kind of man and would never say that he was convinced of something that was not entirely worked out. You can imagine how anxious I am to finish quickly. 271
Bohr had caught a first glimpse of how to stabilize the electrons that orbited with such theoretical instability around Rutherford’s nucleus. Rutherford sent him off to his rooms to work it out. Time was running short; he planned to marry Margrethe Nørland in Copenhagen on August 1. He wrote Harald on July 17 that he was “getting along fairly well; I believe I have found out a few things; but it is certainly taking more time to work them out than I was foolish enough to believe at first. 272I hope to have a little paper ready to show to Rutherford before I leave, so I’m busy, so busy; but the unbelieveable heat here in Manchester doesn’t exactly help my diligence. How I look forward to talking to you!” By the following Wednesday, July 22, he had seen Rutherford, won further encouragement, and was making plans to meet Harald on the way home. 273
Bohr married, a serene marriage with a strong, intelligent and beautiful woman that lasted a lifetime. He taught at the University of Copenhagen through the autumn term. The new model of the atom he was struggling to develop continued to tax him. On November 4 he wrote Rutherford that he expected “to be able to finish the paper in a few weeks.” 274A few weeks passed; with nothing finished he arranged to be relieved of his university teaching and retreated to the country with Margrethe. The old system worked; he produced “a very long paper on all these things.” 275Then an important new idea came to him and he broke up his original long paper and began rewriting it into three parts. “On the constitution of atoms and molecules,” so proudly and bravely titled—Part I mailed to Rutherford on March 6, 1913, Parts II and III finished and published before the end of the year—would change the course of twentieth-century physics. Bohr won the 1922 Nobel Prize in Physics for the work.
* * *
As far back as Bohr’s doctoral dissertation he had decided that some of the phenomena he was examining could not be explained by the mechanical laws of Newtonian physics. “One must assume that there are forces in nature of a kind completely different from the usual mechanical sort,” he wrote then. 276He knew where to look for these different forces: he looked to the work of Max Planck and Albert Einstein.
Planck was the German theoretician whom Leo Szilard would meet at the University of Berlin in 1921; born in 1858, Planck had taught at Berlin since 1889. In 1900 he had proposed a revolutionary idea to explain a persistent problem in mechanical physics, the so-called ultraviolet catastrophe. According to classical theory there should be an infinite amount of light (energy, radiation) inside a heated cavity such as a kiln. That was because classical theory, with its continuity of process, predicted that the particles in the heated walls of the cavity which vibrated to produce the light would vibrate to an infinite range of frequencies.
Читать дальше













