TABLE 1.26 Various constituents of formulated biocidal products
| Ingredient |
Purpose |
Examples |
| Biocide |
Antimicrobial or preservative activity |
QACs, phenolics, biguanides |
| Solvent |
A solvent is a substance (usually liquid) that is capable of dissolving other substances, with water being the most common. Solvents are used for dissolution and dilution of the biocide and other ingredients. |
Deionized water, isopropanol, propylene glycol, urea |
| Emulsifiers, surfactants |
Emulsifers are ingredients (including surfactants) that allow the formation of stable mixtures (emulsions) of water- and oil-soluble ingredients. Surfactants (“surface-acting agents”) can be used as emulsifiers. but also to reduce surface tension, improve wettability of a surface, disperse contaminants, and inhibit foam formation. |
Sodium lauryl sulfate, potassium laurate, lecithin, nonionic and other surfactants (see section 3.16) |
| Thickeners |
A substance used to increase the viscosity of a formulation |
Polyethylene glycol; polysaccharides, like pectin, gums, and alginates |
| Chelating agents or sequestrants |
Binding metals (like calcium and magnesium) and inhibiting their precipitation; water softening and prevention of mineral deposition |
Ethylenediamine, EDTA, EGTA |
| Alkali or acid |
pH stabilization. Alkalis are used as “builders” to optimize the activities of surfactants, including emulsification of soils. Acids are also used to prevent mineral deposition and for water softening. |
Alkalis (NaOH, KOH, silicates); acids (acetic acid, citric acid, phosphoric acid) |
| Buffer |
Maintaining pH over time and increasing alkalinity |
Disodium phosphate |
| Corrosion inhibitors |
Reducing the corrosion rates and protecting the surfaces of metals |
Nitrates, phosphates, molybdates, ethanolamine |
| Others |
Aesthetic qualities |
Colors and fragrances |
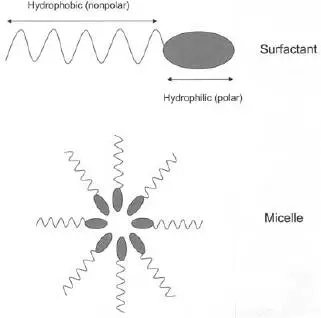
FIGURE 1.26 Basic structures of surfactants and soaps and micelles (a water-in-oil micelle is shown).
Other formulation ingredients, including thickeners, buffers, chelating agents, and fragrances, allow further optimization of the stability, efficacy, and compatibility of the product. Due to the variety of ingredients that can be present in a given formulation, the preservative and/or antimicrobial activity can vary considerably. For this reason, users should pay close attention to the instructions (or labeling) provided with the product for its intended use, including the shelf life, application, dilution (if required), contact time for antimicrobial efficacy, spectrum of activity, safety, and compatibility.
Just as the antimicrobial activity of a biocidal product can vary depending on various formulation effects, it is also affected by various process effects, including variables like temperature, humidity, pressure, time, and biocide concentration. This is particularly true in the development and optimization of processes. Important process variables in various disinfection and sterilization techniques are given in Table 1.27; they are discussed in further detail in chapters 2, 5, and 6.
The activity of a biocide is usually greater as the contact time, temperature, or concentration increases. The effect of contact time is often demonstrated by studying the loss of microbial viability over time, for example, D -value determinations (see section 1.4.2.1). Some biocidal applications are required to be rapid in action due to their practical use, as in the case of hand washing or surface disinfection. In contrast, preservative applications are only required to control microbial growth within a product over a longer exposure time or within a given shelf life. Temperature itself can be a reliable method of disinfection and sterilization, depending on the contact time and temperature for a given application (see section 2.2). In most cases, the activities of chemical biocides are also increased as the temperature increases; however, in the case of higher temperatures, increased degradation can also be observed, depending on the biocide type and the temperature conditions. Various materials or applications may also be restricted to low-temperature exposure conditions, for example, in the case of thermosensitive materials or due to safety concerns.
TABLE 1.27 Examples of process variables in various disinfection/sterilization techniques
| Biocidal process |
Variables |
| Steam (moist heat) |
Temperature, time, pressure, quality of steam (including saturation), biocide penetration |
| Ethylene oxide |
Formulation, temperature, humidity, biocide concentration, vacuum, time, biocide penetration |
| Liquid peracetic acid |
Formulation, temperature, biocide concentration, time, biocide penetration (e.g., directed flow) |
| Hydrogen peroxide vapor |
Temperature, biocide concentration, time, humidity, biocide penetration (vacuum or directed flow) |
| Radiation |
Radiation dose, penetration, exposure time |
Antimicrobial activity is also greater as the concentration of biocide is increased but also varies depending on the biocide and its application. A notable example is alcohols, where less bacteriocidal activity is observed at concentrations greater than 90% alcohol in water, and the optimal range is actually within 60 to 80%; efficacy is dramatically less at lower concentrations. Further, despite the alcohol concentration, little to no efficacy has been reported against bacterial spores. The optimization of a biocide concentration is an important consideration in various disinfection and sterilization processes. Higher biocide concentrations can lead to unwanted effects, including material incompatibility and safety risks, in particular with gas-based applications. In the case of liquid applications, as discussed in section 1.4.6, the efficacy of a biocide can be dramatically enhanced or reduced by various formulation effects that should also be appreciated. These effects include pH (for biocide efficacy and stability), the quality of water, and the presence of excipients, like surfactants.
The control of relative humidity is an important consideration for many gas-based chemical biocidal processes, including the use of ethylene oxide and formaldehyde. Other effects include the state of the biocide (in liquid or gaseous form) and its delivery (to ensure that all site are contacted). Many physical and chemical sterilization processes are conducted under vacuum (e.g., ethylene oxide and plasma-hydrogen peroxide vapor), in vacuum or pressure cycles (e.g., steam), or under specific directed-flow conditions (with liquids and gases) to optimize the penetration of the biocide to all contact sites within a given load.
1.4.8 The Importance of Cleaning
Cleaning is the removal of contamination from an item to the extent necessary for its further processing and its intended subsequent use. In many applications, it is important to ensure the removal of residues following the use of a reusable surface, for example, to prevent cross-contamination between pharmaceutical manufacturing batches, to reduce the level of bioburden on the surface, and particularly, to ensure that a subsequent biocidal process can be effective. Various surfaces require routine cleaning, including manufacturing vessels, equipment, or areas; food-handling surfaces; and reusable medical, veterinary, and dental devices. The presence of various organic (including lipids, proteins, and carbohydrates) and/or inorganic (including various heavy metals like calcium and iron) soils on these surfaces can often dramatically interfere with the activity of a biocide.
Читать дальше













