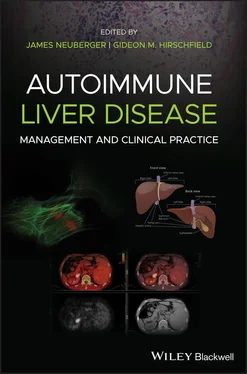
Table 2.1 Comparison of classic autoimmunity with AIH, PBC or PSC.
| Classic autoimmunity |
AIH |
PBC |
PSC |
| Disease‐specific autoantigens |
Yes a |
Yes |
Yes b |
| Disease‐specific autoantibodies |
Yes, types 1 and 2 |
Definite |
Yes |
| Epitope determinant spreading |
Unclear |
No |
Unclear |
| Female predilection |
Yes |
Yes |
No |
| Occurrence in children and adults |
Yes |
No, adults only |
Yes |
| Polygenetic predisposition |
Yes |
Yes |
Yes |
| HLA immunogenetic associations |
Yes |
Yes |
Yes |
| Non‐HLA genetic associations |
Yes |
Yes |
Yes |
| Environmental triggers |
Yes |
Yes |
Yes |
| Tissue/organ‐specific pathology |
Yes |
Yes |
Yes |
| Associated extrahepatic autoimmune diseases |
Yes |
Yes |
Yes |
| Associated IBD |
Yes (weak) |
No |
Yes (strong) |
| Response to immunosuppression |
Yes |
Yes |
No |
| Autoantigen‐specific animal models |
Yes for type 2 No for type 1 |
Yes |
No |
aT and B cell epitopes defined in type 2, not type 1 AIH.
bβ‐Tubulin isoform 5, not rigorously tested to determine prevalence, sensitivity or specificity.
AIH, autoimmune hepatitis; IBD, inflammatory bowel disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Innate immune inflammasome responses, especially those mediated by nucleotide‐binding oligomerization domain (NOD)‐like receptor P3 (NLRP3), occur in both innate immune cells and non‐immune cells, including hepatocytes, cholangiocytes, and hepatic stellate cells (HSCs) [5]. Thus, clinicians must embrace a new paradigm: cells or tissues are not passive “targets” of autoimmune diseases (including AILDs), but instead are active participants in the immunopathogenesis of their own injury and demise.
The innate immune system responds immediately to PAMPs, comprising microbial constituents, and to DAMPs, comprising cellular constituents from stressed, neoplastic or dying cells ( Table 2.2). Both PAMPs and DAMPs bind to pattern recognition receptors (PRRs) and other receptors expressed on neutrophils, dendritic cells (DCs), activated macrophages (including Kupffer cells), and natural killer (NK) and natural killer T (NKT) cells [3]. NK cells express killer receptors for stressed, dying or neoplastic cells and Fc receptors that mediate antibody‐dependent cell‐mediated cytotoxicity (ADCC) of target cells coated with antibodies [3]. Antigen‐presenting cells (APCs) activate NKT and γδT cells by invariant HLA class I‐like molecule (CD‐1) presentation of lipid or glycolipid antigens [3]. γδT cells protect from autoimmune diseases and microbial infections and participate in mucosal immunity and tumor surveillance.
Table 2.2 Comparison of selected features of innate and adaptive immunity.
|
Innate immunity |
Adaptive immunity |
| Characteristics |
| Onset |
Rapid responses mediated by preformed PRRs for microbial PAMPs and DAMPs |
Delayed due to requirement for antigen activation, clonal proliferation and maturation of effector cell functions |
| Specificity |
PAMPs, DAMPs, ROS, activated C′ proteins, apoptotic bodies |
Epitopes of peptide antigens recognized by TCRs or B‐cell immunoglobulins |
| Genetics |
Restricted, germline‐encoded |
Complex with TCRs and antigen‐binding domains of immunoglobulins produced by somatic recombination of gene segments |
| Diversity |
Limited Evolutionarily conserved |
Virtually infinite due to TCR rearrangement |
| Memory |
No |
Yes. Memory T‐ and B‐cell responses capable of anamnestic reactivation |
| Self‐tolerance |
Discriminates pathogens and endogenous DAMPs. Not responsive to autoantigens |
Positive and negative selection of TCR and B‐cell immunoglobulin reduce autoreactive capacity |
| Components |
| Physical barriers |
Skin, mucosal epithelia of oropharynx, gut and reproductive tract, antimicrobial proteins |
Intraepithelial lymphocytes within gut, Peyer's patches |
| Cells |
Dendritic cells, monocytes, macrophages (Kupffer cells), neutrophils, NK cells, NKT cells |
Professional APCs, αβT cells, γδT cells, natural and inducible Treg cells, B cells, plasma cells |
| Proteins |
C′ proteins, IFN‐α, ‐β, ‐γ, cytokines, chemokines |
T‐cell cytokines IgM, IgG, IgA, IgE antibodies |
| Role of liver as an immunologic organ |
Yes DCs, neutrophils, Kupffer cells, excessive concentrations of NK cells, NKT cells IFN‐α, ‐β, ‐γ, balance between proinflammatory and immunosuppressive chemokines |
Yes Antigen presentation by Kupffer cells, LSECs, HSCs, cytokines stimulate hepatocytes and cholangiocytes, PD‐L1/2 induce senescent T‐cell apoptosis and inhibition of activated CD8 T cells |
C′, complement; DAMPs, damage‐associated molecular patterns; HSC, hepatic stellate cell; IFN, interferon; LSEC, liver sinusoidal endothelial cell; NK, natural killer; PAMPs, pathogen‐associated molecular patterns; PD‐L1/2, programmed death ligands 1 and 2; PRRs, pattern recognition receptors; ROS, reactive oxygen species; TCR, T cell receptor.
Examples of common PAMPs: (i) lipopolysaccharide (LPS), a cell wall constituent of all Gram‐negative bacteria; (ii) lipotechoic acid, a cell wall constituent of all Gram‐positive bacteria; (iii) peptidoglycans, essential cell wall components of all bacteria; (iv) viral proteins; and (v) unmethylated bacterial CpG dinucleotide motifs.
Examples of common DAMPs: (i) nuclear proteins; (ii) cytosolic proteins and lysosomal enzymes; and (iii) non‐proteins, such as ATP, DNA, RNA, mitochondrial DNA, U1 ribonucleoprotein (U1RNP) and uric acid.
Microbial PAMPs bind predominantly to PRRs called Toll‐like receptors (TLRs). DAMPs bind to PRRs and receptors for chemical ligands or nuclear constituents. Still other innate receptors bind apoptotic bodies and activated complement (C′) molecules. PAMPs and/or DAMPs activate DCs and macrophages (including Kupffer cells), increasing phagocytic activity and inducing secretion of chemokines and proinflammatory cytokines interleukin (IL)‐1β, IL‐6, IL‐12, and IL‐18 and tumor necrosis factor (TNF)‐α, as well as the immunosuppressant cytokine IL‐10 [3]. The relative amounts of specific PAMPs, DAMPs, cytokines and chemokines creates a dynamic balance between proinflammatory and immunosuppressive cytokines that determines whether the adaptive immune response promotes immunopathology ( Figure 2.2).
Liver as an Innate Immune Organ
The liver is an essential innate immune organ that must either tolerate or respond to microbial PAMPs, toxins, xenobiotics, food antigens, microbial pathogens, and neoplastic cells [6]. The liver contains specialized liver sinusoidal endothelial cells (LSECs) and large quantities of Kupffer cells, DCs and NK, NKT and γδT cells. Most intrahepatic B cells are B1 cells, an innate B‐cell subset that secretes low‐affinity IgM antibodies against glycoproteins, called “natural antibodies.”
The liver also synthesizes C′ proteins, acute‐phase reactant proteins, and multiple cytokines. PAMP and DAMP activation of TLRs on Kupffer cells, LSECs, hepatocytes, DCs and HSCs primarily generates IL‐10, providing an immunosuppressive environment. However, high quantities of PAMPs or DAMPs induce the proinflammatory cytokines IL‐1β, IL‐6, IL‐12, IL‐18 and TNF‐α. HSCs normally produce matrix proteins, but once transformed into myofibroblasts they secrete collagen, resulting in fibrosis. PAMPs and DAMPs also induce NLRP3 inflammasome responses [5] in hepatocytes, cholangiocytes and HSCs, resulting in their secretion of proinflammatory cytokines and chemokines.
Читать дальше