Figure 13
The third scenario is more subtle. A patient comes to the hospital with a high fever. He has two sets of blood cultures drawn in the emergency department. Two days later, S. epidermidis is recovered from one of these blood culture sets. As with S. aureus , this organism may show resistance to a variety of antimicrobial agents that are used to treat infected patients. However, no susceptibility testing is done by the laboratory, and this practice is acceptable to the clinician caring for the patient. Why? S. epidermidis is a component of skin microbiota and may have contaminated the culture. If the laboratory had performed the susceptibility testing without considering that this isolate was a potential contaminant, they would be validating that the isolate was clinically significant. In this setting, the laboratory should only do susceptibility testing if instructed to by the caregiver, who is in a better position to know if this organism is clinically important.
There are several approaches to antibacterial susceptibility testing. All the approaches are highly standardized to ensure that the susceptibility results will be consistent from laboratory to laboratory. Screening of selected organisms for resistance to specific antimicrobial agents is one strategy that is frequently used, especially with the emergence of resistance in three organisms: S. aureus to cefoxitin to predict oxacillin resistance, S. pneumoniae to penicillin, and Enterococcus faecium and Enterococcus faecalis to vancomycin. Other strategies are to determine susceptibility to a preselected battery of antimicrobial agents using automated or manual systems that determine the MIC of antibiotics to the organism being tested or by using the disk diffusion susceptibility testing technique.
A novel approach to susceptibility testing is to perform MIC determinations using the E-test. The E-test is a plastic strip that contains a gradient of a specific antimicrobial agent. This strip is applied to a lawn of bacteria on an agar plate. Where the zone of inhibition intersects with the strip is the MIC value of that antibiotic for the organism tested. This test has many applications but is used most frequently for determining penicillin MIC values for S. pneumoniae isolates that show resistance to penicillin in the screening test previously described ( Fig. 14).
Susceptibility testing is performed with increasing frequency on Candida spp. other than C. albicans but is rarely done on other yeasts and almost never on molds. Because of their slow growth, special susceptibility testing techniques are used for the mycobacteria.
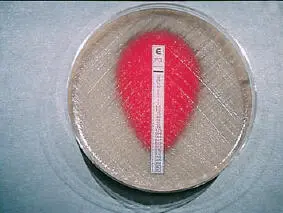
Figure 14
Tissue culture for Chlamydia and viruses
Both Chlamydia , a bacterium, and the viruses are obligate intracellular parasites. As such, they do not grow on artificial media, as fungi and other bacteria do. Rather, they can only grow by parasitizing living animal cells (including human cells) that are maintained by continuous tissue culture. Animals such as mice, or chicken eggs, can be inoculated in an attempt to isolate certain viruses, but this approach is rarely done. Tissue culture for Chlamydia may still be attempted, especially in situations where the detection of C. trachomatis is at issue in a legal proceeding, such as a case of sexual abuse of a child. However, molecular detection has become the standard method for diagnosis of C. trachomatis infection.
Tissue culture is still an important technique for the detection of viruses in many laboratories, though laboratories are converting to molecular methods for viral detection at an increasing rate. Herpes simplex virus can be isolated from skin and genital tract lesions, often within the first 24 hours of incubation. Another herpesvirus, varicella-zoster virus, the etiologic agent of chicken pox and herpes zoster, can also be isolated from skin lesions, but it typically requires 3 to 7 days to grow. The enteroviruses are the major etiologic agents of aseptic meningitis and can be isolated from cerebrospinal fluid, but at a significantly reduced rate compared with molecular detection.
A modification of the tissue culture technique is done to detect cytomegalovirus and several respiratory viruses in clinical specimens called rapid centrifugation cultures or shell vial cultures. In this method, the specimen is centrifuged onto tissue culture cells that are growing on a round glass coverslip inside a vial referred to as a shell vial. The cells are incubated for a brief period of time (24 to 72 hours) and then stained with fluorescent antibodies to detect the virus. This technique is much more rapid and sensitive than conventional tissue culture but is still less sensitive than molecular detection.
It is not always possible to isolate a microorganism by culture, visualize it microscopically, or detect it by antigenic or molecular detection techniques. In those situations, an alternative approach is to determine if the patient has mounted an immune response against a specific agent as evidence that he or she has been infected with that agent. The immune response is generally measured by detecting antibodies in the serum of patients—thus the name serology.
Serology has both advantages and disadvantages. As advantages, (i) specimens for testing are readily available; (ii) antibodies are relatively stable molecules, so transport is not a major concern as it is with culture; and (iii) tests have been designed that can detect most known agents, such as HIV and HCV, which are difficult to detect by other means. Depending on the target antigen against which the immune response is measured, the test can show both high sensitivity and high specificity. Compared with other techniques, these tests are relatively inexpensive and easy to perform, in part because they have been automated. As a result, they can be used to screen large numbers of specimens for selected infectious agents. For example, this approach is used to screen blood products used for transfusions to ensure that the transfused patient does not receive blood contaminated with hepatitis B and C viruses, HIV, or T. pallidum , the agent of syphilis.
Serologic tests also have several disadvantages and should be interpreted with some caution. To have a positive test, the patient must have mounted an immune response. Serum obtained from an acutely ill patient may have been taken during the window period in an infection before the patient had time to mount an immune response. Therefore, to get the most accurate result, acuteand convalescentspecimens should be obtained. The convalescent specimen should show a significant increase (or, in some cases, decrease) from the antibody level of an acute specimen. This is usually a 4-fold change in the titer. Because the convalescent specimen should be obtained a minimum of 2 weeks after the acute specimen, serologic diagnosis is often retrospective. Because obtaining a convalescent specimen is often difficult logistically, the only value that may be available is that from the acute specimen. Patients may have relatively high antibody levels because of previous infection with the test organism and, as a result, may have a false-positive result. Antigenic cross-reactions between the test organism and other antigens may also lead to false-positive results. Some immunocompromised patients are unable to mount a response and may never have a positive serologic test.
Читать дальше














