The source of both organic and inorganic arsenicals are naturally occurring minerals, such as, arsenopyrite (FeAsS), realgar (As 4S 4) and orpiment (As 2S 3). As these erode, they react with moisture and oxygen to form arsenites and arsenates that are water soluble and consequently end up in both surface and groundwater. Some of these chemical forms and oxidation states cause acute and chronic adverse health effects, including cancer (Hughes, 2002). The metabolism involves reduction to a trivalent state and oxidative methylation to a pentavalent state. The trivalent arsenicals, including those methylated, have more potent toxic properties than the pentavalent arsenicals. The exact mechanism of the action of arsenic is not known, but several hypotheses have been proposed. What is missing in this analysis is the role of artificial chemicals. At a biochemical level, inorganic arsenic in the pentavalent state may replace phosphate in several reactions. In the trivalent state, inorganic and organic (methylated) arsenic may react with critical thiols in proteins and inhibit their activity. However, this ‘organic’ in New Science doesn’t mean that an artificial state has been avoided. As such, potential mechanisms include genotoxicity, altered DNA methylation, oxidative stress, altered cell proliferation, co-carcinogenesis, and tumor promotion cannot be tracked to artificial chemicals. A better understanding of the mechanism(s) of action of arsenic will make a more confident determination of the risks associated with exposure to this chemical.
In surface waters, these chemicals can be absorbed by algae that then convert them to arsenosugars, arsinolipids and arsenobetaine. In surface waters, these can be absorbed by algae that then convert them to arsenosugars, arsinolipids and arsenobetaine. Fish and other forms of marine life feed on these algae and concentrate the arsenic compounds. When the same arsenic compounds are absorbed by plants, similar but less complex reactions take place and further dilution occurs when they are passed on to grains.
Figure 2.5 Shows the pathway followed by the original naturally occurring ore, containing arsenic. Most arsenic in the terrestrial environment is found in rocks and soils. Arsenic in surface and ground water is mostly a mixture of arsenite and arsenate. Although New Science designates various components in molecular form, in reality molecules are fictitious and never exist in isolation. During the pre-New Science era chemical equations were not written in molecular or atomic form, hence the words, such as ‘air’ (instead of Oxygen), ‘moisture’ (instead of H 2O) and chosen.
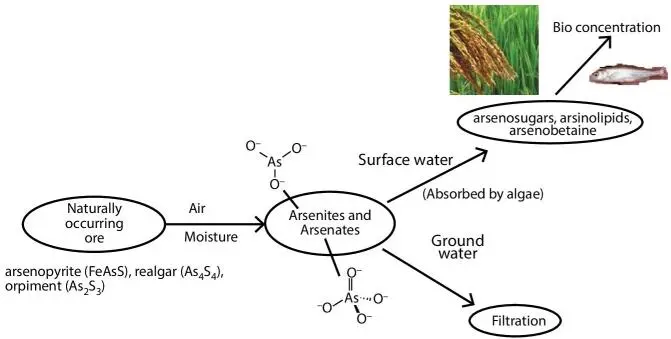
Figure 2.5Pathway followed by arsenic chemicals.
This figure shows that in order for arsenic to travel natural pathway, the entire chain of air and moisture has to be free of synthetic chemicals. In the post industrial revolution, major sources of arsenic include the combustion of coal, nonferrous metal smelting, and the burning of agricultural wastes. These are inherently toxic to the environment. Similarly, each chemical containing arsenic that has been widely used as herbicides, fungicides, wood preservatives, desiccants, cattle and sheep dips, and dyestuffs is necessarily synthetic or artificially processed. Today, arsenic continues to be widely used in agriculture, in glass and ceramics, as a metal alloy, and in semiconductors and other electronic devices – all causing irreparable harm to the environment. It is no surprise that the entire branch of toxicology deals with only artificial type of arsenic products (Hughes, 2002).
2.2.5 Refining Techniques
In terms of processing of petroleum crude, Al-Rāzī’s work is likely the oldest complete reference available today. In his Kitāb al-Asār , Al-Rāzī described two methods for the production of kerosene, termed naft abyad (white petroleum), using an apparatus called an alembic. Picture 2.1 shows this device. The complete distilling apparatus consists of three parts (Bearman et al ., 2012):
1 the “cucurbit” (Arabic, qar‘; Greek, βίκος, bikos), the still pot containing the liquid to be distilled
2 The “head” or “cap” (Arabic, al-anbīq; from Greek ἄμβιξ, ambix, meaning `cup, beaker`) fits over the mouth of the cucurbit to receive the vapors,
3 A downward-sloping “tube” (Greek σωλήν, sōlēn), leading to the “receiver” (Arabic, kābīlā, Greek ἄγγος, angos, or φιάλη, phialē) container.
This set up is often reduced to one retort, used for distillation. This setup, however, uses open fire and the material used in different parts is entirely sustainable, it has no artificial material in it. The original process was used to prepare rose water.
One method used clay as an absorbent, whereas the other method used ammonium chloride (sal ammoniac). The distillation process was repeated until most of the volatile hydrocarbon fractions had been removed and the final product was perfectly clear and safe to burn. It is not clear from the literature what was the most used source for producing kerosene, but the word naft implies a petroleum source. However, it is conceivable similar technique was used to refine olive oil, which would in fact produce gases that are beneficial to human health (Islam et al. , 2010). During the same period, kerosene was also produced during the same period from oil shale and bitumen by heating the rock to extract the oil, which was then distilled.
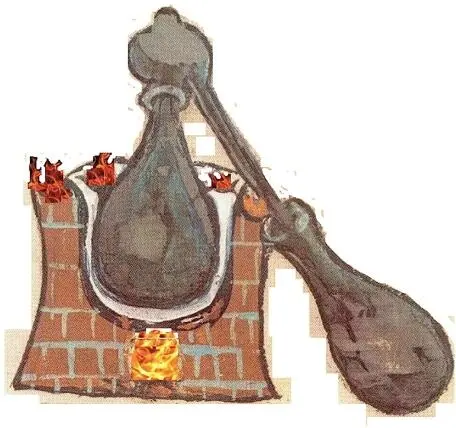
Picture 2.1The refining technique used by the Alchemists.
Similarly, Avicenna wrote volumes on plants and their uses. His instruction manual also contained refining processes. His improvement of the cooling system within the distillation apparatus is most noteworthy (Waines, 2010).
Today, such distillation processes are all be eliminated. Perhaps the closest to retaining the original sustainable refining technologies is the perfume industry, for which extracting essential oils from plants is the biggest technological challenge. The advantage of distillation is that the volatile components can be distilled at temperatures lower than the boiling points of their individual constituents and are easily separated from the condensed water. For the perfume industry, the use of water is desirable as water is the most ubiquitous material and does not alter the original aroma. Such fascination for water is absent in the chemical industry, particularly the ones dealing with petroleum fluids. In fact, in considering petroleum waste disposal, water is considered to be an undesirable by-product of the petroleum operation that need to be removed in order to ensure proper functioning of the refining process.
Similarly, the process of expression, also referred to as cold pressing, is popular in the perfume industry. Numerous essential oils are routinely extracted through cold pressing. In particular, citrus essential oils, such as tangerine, lemon, bergamot, sweet orange, and lime employ the process of expression. In older times, expression was done in the form of sponge pressing. The zest or rind of the citrus would first be soaked in warm water to make the rind more receptive to the pressing process. A sponge would then be used to press the rind, thus breaking the essential oil cavities, and absorb the essential oil. Once the sponge was filled with the extraction, it would then be pressed over a collecting container, and there it would stand to allow for the separation of the essential oil and water/juice. The essential oil would finally be siphoned off. Centuries ago, less labor-intensive processes have been employed. One such process, termed the Écuelle à piquer , involves a prodding, pricking, sticking action to release the essential oil. During this process, the rind of the fruit is placed in a container having spikes that will puncture the peel while the device is rotated. The puncturing of the rind will release the essential oil that is then collected in a small area below the container. While it is not commonly understood, the material used in those puncturing spikes would affect both the quality of the essential oil and the sustainability of the process (Khan and Islam, 2016). Today, the majority of modern expression techniques are accomplished by using machines using centrifugal force. The spinning in a centrifuge separates the majority of essential oil from the fruit juice.
Читать дальше














