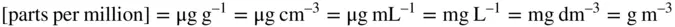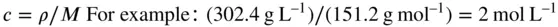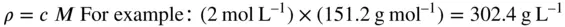2 2Flanagan RJ, Taylor A, Watson ID, Whelpton R. Fundamentals of Analytical Toxicology. Chichester: Wiley, 2007
This book is intended for use by scientists trained appropriately in laboratory work. Care should be taken to ensure the safe handling of all chemical and biological materials, and particular attention should be given to the possible occurrence of allergy, infection, fire, explosion, or poisoning (including transdermal absorption or inhalation of toxic vapours). Readers are expected to consult current local health and safety regulations and to adhere to them.
Nomenclature, Symbols, and Conventions
We have followed IUPAC nomenclature for chemical names except when Chemical Abstracts nomenclature or trivial names are more readily understood. With regard to symbols, we have adopted the convention that variables and constants are italicized, but labels and mathematical operators are not. Thus, for example, the acid dissociation constant is written K a, K being the variable, a being a label to denote that it is an acid dissociation constant. The notation for the negative logarithm of K ais p K a– p is a mathematical symbol and is not italicized. Where the subscript is a variable then it is italicized, so the concentration at time t , is C t, but the concentration at time 0 is C 0. Note especially that relative molecular mass (molecular weight, relative molar mass), the ratio of the mass of an atom or molecule to the unified atomic mass unit (u), is referred to throughout as M r. The unified atomic mass unit, sometimes referred to as the dalton (Da), is defined as one twelfth of the mass of one atom of 12C. The symbol amu for atomic mass unit can sometimes be found, particularly in older works. The unified atomic mass unit is not a Système International (SI) unit of mass, although it is (only by that name, and only with the symbol u) accepted for use with SI.
As to drugs and pesticides, we have used recommended International Non-proprietary Name (rINN) or proposed International Non-proprietary Name (pINN) whenever possible. For misused drugs, the most common chemical names or abbreviations have been used. It is worth noting that for rINNs and chemical nomenclature, it is now general policy to use ‘f’ for ‘ph’ (e.g. sulfate not sulphate), ‘t’ for ‘th’ (e.g. chlortalidone not chlorthalidone) and ‘i’ for ‘y' (mesilate not mesylate for methanesulfonate, for example). However, so many subtle changes have been introduced that it is difficult to ensure compliance with all such changes. Names that may be encountered include the British Approved Name (BAN), the British Pharmacopoeia (BP) name, the United States Adopted Name (USAN), the United States National Formulary (USNF) name, and the United States Pharmacopoeia (USP) name. Where the rINN is markedly different from common US usage, for example acetaminophen rather than paracetamol, meperidine instead of pethidine, the alternative is given in parentheses at first use and in the index.
Isotopically-labelled compounds are indicated using the usual convention of square brackets to denote the substituted atoms, and site of substitution where known. For example, [ 2H 3- N -methyl]-hyoscine indicates that the hydrogen atoms in the N -methyl group have been substituted by deuterium – this should not be confused with N -methylhyoscine (methscopolamine).
A useful source of information on drug and poison nomenclature is the Merck Index Online ( www.rsc.org/Merck-Index/). Chemical Abstracts Service (CAS) Registry Numbers (RN) provide a unique identifier for individual compounds, but it is important to note that salts, hydrates, racemates, etc., each have their own RNs. Similarly, when discussing dosages we have tried to be clear when referring to salts, and when to free acids, bases, or quaternary ammonium compounds.
The oxidation number of metal ions is given by, for example, iron(II), but older terminology such as ferrous and ferric iron for Fe 2+and Fe 3+, respectively, will be encountered in the literature.
We emphasize that cross-referral to an appropriate local or national formulary is mandatory before any patient treatment is initiated or altered. Proprietary names must be approached with caution – the same name is sometimes used for different products in different countries.
Uniform Resource Locators
Uniform resource locators (URLs, web addresses) were correct at the time of printing. If the cited links are broken, readers should use an appropriate search engine or other resource to find the current URL unless directed otherwise.
Amount Concentration and Mass Concentration
In parts of Europe some laboratories report analytical toxicology data in ‘amount concentration’ using what have become known as SI molar units (μmol L –1, etc.), whilst others, especially in the US, continue to use mass concentration [so-called ‘traditional’ units (mg L –1, etc. or even mg dL –1)]. Most published analytical toxicology and pharmacokinetic data are presented in SI mass units per millilitre or per litre of the appropriate fluid [the preferred unit of volume is the litre (L)], or units that are numerically equivalent in the case of aqueous solutions:
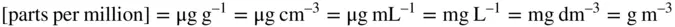
When preparing written statements for a court of law or other purpose outside the normal reporting channels it is advisable to write out the whole unit of measurement in full (milligrams per litre, for example).
An exception to the above is carboxyhaemoglobin saturation (COHb), which is usually reported as a percentage of the total haemoglobin present in the sample (% COHb) – the SI convention is that fractions of one should be used rather than percentages, but this is generally ignored.
We have followed the recommendations of the UK NPIS/ACB (National Poisons Information Service/Association for Clinical Biochemistry and Laboratory Medicine) for reporting analytical toxicology results and have used the litre as the unit of volume and SI mass units except for lithium (and sometimes toxic metals/trace elements), methotrexate, and thyroxine (NPIS/ACB. Laboratory analyses for poisoned patients: Joint position paper. Ann Clin Biochem 2002; 39: 328–39). More information on SI is available (Flanagan RJ. SI units – Common sense not dogma is needed. Br J Clin Pharmacol 1995; 39: 589–94).
Conversion from mass concentration ( ρ ) to amount concentration ( c ) (‘molar units’) and vice versa is simple if the molar mass ( M ) of the compound of interest is known. Thus, if a solution contains 302.4 g L –1of a compound of M r151.2 g mol –1
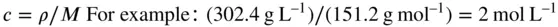
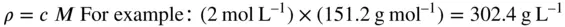
However, such conversions always carry a risk of error. Especial care is needed in choosing the correct M rif the drug is supplied as a salt, hydrate, etc. This can cause great discrepancies especially if the contribution of the accompanying anion or cation is high. Most analytical measurements are reported in terms of the free acid or base, and not the salt.
We thank Drs Andrew Taylor and Ian Watson for contributions incorporated from Edition 1 of this book, Dr Lewis Couchman, Dr Natalia Kroupina, Prof John Langley, Ms Karen Morgan, Dr Simon Nelms, Prof Fritz Pragst, Ms Annie Sanger, Mr Peter Streete, and Dr Nicholas Tiscione for their help, and Prof Olaf Drummer and Prof Ilkka Ojanperä for commenting on drafts of the manuscript. We also thank Ms J Cossham (Wiley) for her help and encouragement.
Читать дальше