Jane Flint - Principles of Virology, Volume 1
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology, Volume 1» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology, Volume 1
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Principles of Virology, Volume 1: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology, Volume 1»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology, Volume 1 — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology, Volume 1», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
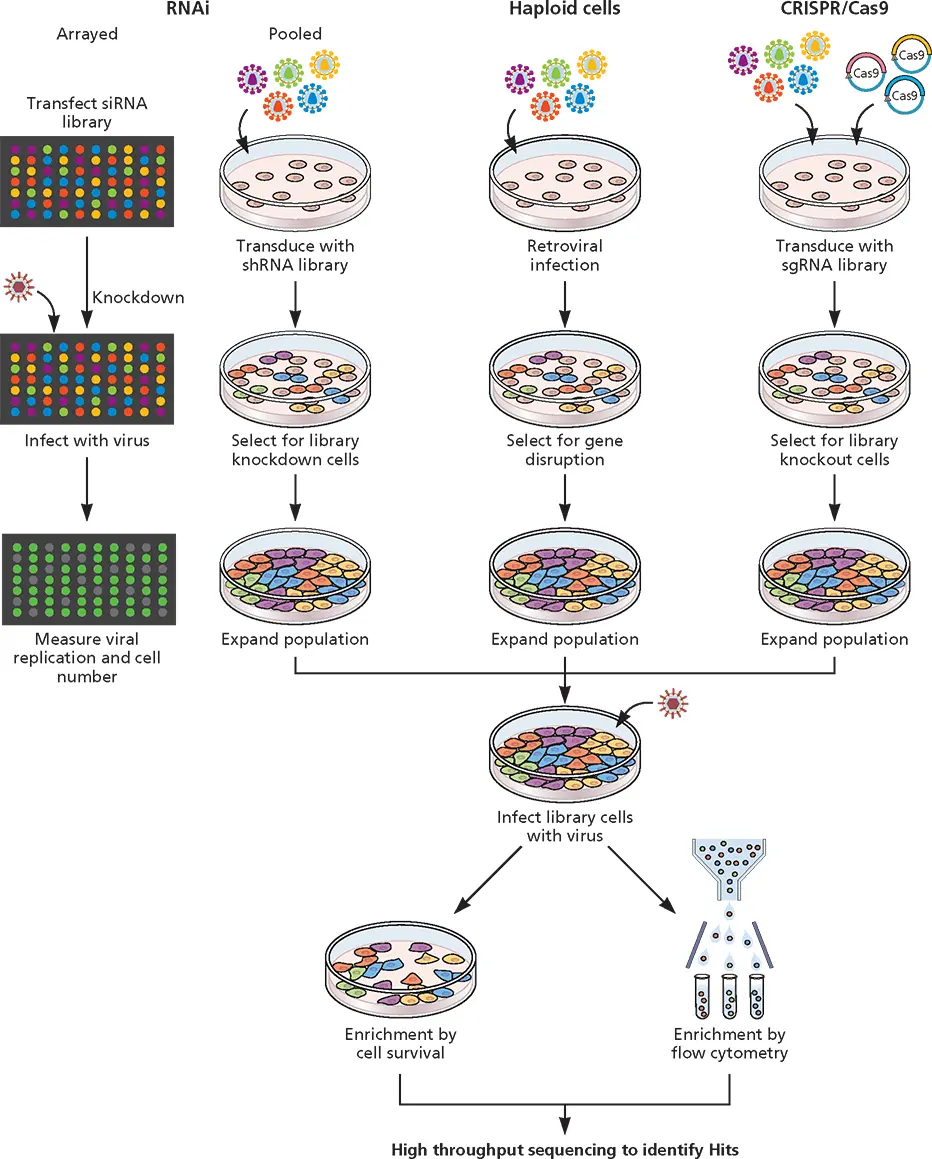
Figure 3.13 Use of RNAi, haploid cells, and CRISPR-Cas9 to study virus-host interactions. In arrayed screens, siRNAs are introduced into cells growing in wells that are subsequently infected with virus. Production of infectious virus or a viral protein is quantified by plaque assay or measurement of a fluorescent protein. Individual siRNA with the desired effect can be identified based on their location in the multiwell plate. In pooled RNAi screens, collections of shRNA producing lentiviral vectors are used to infect cells. After selection for cells with integrated vectors, the cells are infected with the test virus and the production of a viral protein or infectious virus is monitored. In pooled haploid cell screens, cells are infected with lentiviruses at a low multiplicity of infection so that on average one viral genome integration per cell takes place. In pooled CRISPR-Cas9 screens, libraries of sgRNAs are introduced, via lentivirus vector, into cells that produce Cas9. After selection for lentiviral integration, cells are infected with virus. Cell survival and production of infectious virus or a viral protein may be measured depending on what types of genes are sought (e.g., those that are essential for reproduction). In each screen, the cell gene that is disrupted is identified by nucleotide sequencing.
While the experimental use of RNAi can lead to reduced protein production, genomic manipulation by CRISPR-Cas9 has advantages of complete depletion of the protein through the production of a homozygous null genotype and fewer off-target effects. With CRISPR-Cas9, the expression of a gene can be permanently extinguished. In contrast, the shRNA-expressing provirus must continually silence the product of ongoing transcription.
Haploid Cell Screening
Haploid cell lines have been used to identify genes required for viral reproduction. These cells, which have only one copy of each chromosome, are infected with retroviruses under conditions where one integration event occurs per cell. The disruption of individual genes that are essential for viral replication can be identified by the isolation of cells resistant to infection ( Fig. 3.13). Surviving cells are expanded and the site of proviral integration is determined by PCR and high-throughput sequencing. This approach has been used to identify receptors for viruses, including ebolavirus, Lassa virus, and hantavirus, and genes required for receptor modification and endosomal trafficking.
While powerful, a drawback of this approach is that only a few haploid cell lines are available, and not all viruses can infect these cells.
Engineering Viral Genomes: Viral Vectors
Naked DNA can be introduced into cultured animal cells as complexes with calcium phosphate or lipid-based reagents or directly by electroporation. Such DNA can direct synthesis of its gene products transiently or stably from integrated or episomal copies. Introduction of DNA into cells is a routine method in virological research and is also employed for certain clinical applications, such as the production of a therapeutic protein or a vaccine or the engineering of primary cells, progenitor cells, and stem cells for subsequent introduction into patients. However, this approach is not suitable for all applications. In some cases, gene delivery by viral vector is preferred. Viral vectors have also found widespread use in the research laboratory, including applications in which the delivery of a gene to specific cells, or at high efficiency, is desired. The use of viral vectors for gene therapy, the delivery of a gene to patients who either lack the gene or carry defective versions of it, or to destroy tumors typically employs viral vectors, not naked DNA (see Volume II, Chapter 9). In one application, DNA including the gene is introduced and expressed in cells obtained from the patient. After infusion into patients, the cells can become permanently established. If the primary cells to be used are limiting in a culture (e.g., stem cells), it is not practical to select and amplify the rare cells that receive naked DNA. Recombinant viruses carrying foreign genes can infect a greater percentage of cells and thus facilitate generation of the desired population. A complete understanding of the structure and function of viral vectors requires knowledge of viral genome replication, a topic discussed in subsequent chapters for selected viruses and summarized in the Appendix.
Design requirements for viral vectors include the use of an appropriate promoter, maintenance of genome size within the packaging limit of the particle, and elimination of viral virulence, the capacity of the virus to cause disease. Expression of foreign genes from viral vectors may be controlled by homologous or heterologous promoters and enhancers chosen to support efficient or cell-type-specific transcription, depending on the goals of the experiment. Such genes can be built directly into the viral genome or introduced by recombination in cells, as described above (see “Engineering Mutations into Viral Genomes”). The viral vector genome generally carries deletions and sometimes additional mutations. Deletion of some viral sequences is often required to overcome the limitations on the size of viral genomes that can be packaged in virus particles.
When viral vectors are designed for therapeutic purposes, it is essential to prevent their reproduction as well as destruction of target host cells. The deletions necessary to accommodate a foreign gene may contribute to such disabling of the vector. For example, the E1A protein-coding sequences that are always deleted from adenovirus vectors are necessary for efficient transcription of viral early genes; in their absence, viral yields from cells in culture are reduced by about 3 to 6 orders of magnitude (depending on the cell type). Removal of E1A-coding sequences from adenovirus vectors is therefore doubly beneficial, although it is not sufficient to ensure that the vector cannot reproduce or induce damage in a host animal. Adenovirus-associated virus vectors are not lytic, obviating the need for such manipulations. As discussed in detail in Volume II, Chapter 9, production of virus vectors that do not cause disease can be more difficult to achieve.
A summary of viral vectors is presented in Table 3.1, and examples are discussed below.
DNA Virus Vectors
One goal of gene therapy is to introduce genes into terminally differentiated cells. Such cells normally do not divide, and they cannot be propagated in culture. Moreover, the organs they comprise cannot be populated with cells infected by viruses ex vivo . DNA virus vectors have been developed to overcome some of these problems.
Table 3.1 Some viral vectors
| Virus | Insert size | Integration | Duration of expression | Advantages | Potential disadvantages |
|---|---|---|---|---|---|
| Adeno-associatedvirus | ~5 kb | No | Long | Nonpathogenic, episomal, infects nondividing and dividing cells, broad tropism, low immunogenicity | Small transgene capacity, helper virus needed for vector production |
| Adenovirus | ~8–38 kb | No | Short | Broad tropism, efficient gene delivery, infects nondividing and dividing cells, large cargo capacity | Transient, immunogenic, high levels of preexisting immunity |
| Baculovirus | No known upper limit | No | Short | High levels of protein synthesis, recombinant viruses easily made, more than one protein can be made in same cells | Insect cells typically used, no replication in mammalian cells, human type protein glycosylation not 100% efficient, paucimannose structures present |
| Gammaretroviru s (murine leukemia virus) | 8 kb | Yes | Short | Stable integration, broad tropism possible via pseudotyping, low immunogenicity, low preexisting immunity | Risk of insertional mutagenesis, poor infection of nondividing cells, faulty reverse transcription |
| Herpes simplex virus | ~50 kb | No | Long in central nervous system, short elsewhere | Infects nondividing cells, large capacity, broad tropism, latency | Virulence, persistence in neurons, high levels of preexisting immunity, may recombine with genomes in latently infected cells |
| Lentivirus | 9 kb | Yes | Long | Stable integration, transduces nondividing and dividing cells | Potential insertional mutagenesis; none detected in clinical trials |
| Rhabdovirus | ~4.5 kb | No | Short | High-level expression, rapid cell killing, broad tropism, lack of preexisting immunity | Virulence, highly cytopathic, neurotropism, immunogenic |
| Vaccinia virus | ~30 kb | No | Short | Wide host range, ease of isolation, large capacity, high-level expression, low preexisting immunity | Transient, immunogenic |

Figure 3.14 Adenovirus vectors. High-capacity adenovirus “gutless” vectors contain only the origin-of-replication-containing inverted terminal repeats (ITR), the packaging signal (blue arrows), the viral E4 transcription unit (red arrow), and the transgene with its promoter. Additional DNA flanking the foreign gene must be inserted to allow packaging of the viral genome (not shown). A helper virus (bottom) is required to package the recombinant vector genome. Two loxP sites for cleavage by the Cre recombinase have been introduced into the adenoviral helper genome (red arrowheads). Infection of cells that produce Cre leads to excision of sequences flanked by the loxP sites so that the helper genome is not packaged.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology, Volume 1»
Представляем Вашему вниманию похожие книги на «Principles of Virology, Volume 1» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology, Volume 1» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.



