Jane Flint - Principles of Virology, Volume 1
Здесь есть возможность читать онлайн «Jane Flint - Principles of Virology, Volume 1» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Principles of Virology, Volume 1
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Principles of Virology, Volume 1: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Principles of Virology, Volume 1»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume I: Molecular Biology
Volume II: Pathogenesis and Control
Principles of Virology, Fifth Edition
Principles of Virology, Volume 1 — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Principles of Virology, Volume 1», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
(ii) (– ) strand RNA viruses.Genomic RNA of (–) strand RNA viruses is not infectious, because it can be neither translated nor copied into (+) strand RNA by host cell RNA polymerases ( Chapter 6). Two different experimental approaches have been used to develop infectious DNA clones of these viral genomes ( Fig. 3.12B and C).
The recovery of influenza virus from cloned DNA is achieved using an expression system in which cloned DNA copies of the eight RNA segments of the viral genome are inserted between two cellular promoters, so that complementary RNA strands can be synthesized ( Fig. 3.12B). When all eight plasmids carrying DNA for each viral RNA segment are introduced into cells, infectious influenza virus is produced.
When the full-length (–) strand RNA of viruses with a nonsegmented genome, such as vesicular stomatitis virus (a rhabdovirus), is introduced into cells containing plasmids that produce viral proteins required for production of mRNA, no infectious virus is recovered. Lack of infectivity is thought to be a consequence of the hybridization of fulllength (–) strand RNA with (+) strand mRNAs produced from plasmids encoding viral proteins. Such hybridization might interfere with association of the (–) strand RNA with the N protein, which is required for copying by the viral RNA-dependent RNA polymerase. In contrast, when a fulllength (+) strand RNA is transfected into cells that have been engineered to synthesize the vesicular stomatitis virus nucleocapsid protein, phosphoprotein, and polymerase, the (+) strand RNA is copied into (–) strand RNAs. These RNAs initiate an infectious cycle, leading to the production of new virus particles.
dsRNA viruses.Genomic RNA of dsRNA viruses is not infectious because ribosomes cannot access the (+) strand in the duplex. The recovery of reovirus from cloned DNA is achieved by an expression system in which cloned DNA copies of the 10 RNA segments of the viral genome are inserted under the control of a promoter for bacteriophage T7 RNA polymerase ( Fig. 3.12D). When all 10 plasmids carrying DNA for each viral dsRNA segment are introduced into cells, infectious reovirus is produced.
Types of Mutation
Recombinant DNA techniques allow the introduction of many kinds of mutation at any desired site in cloned DNA ( Box 3.10). Deletion mutationscan be used to remove an entire gene to assess its role in reproduction, to produce truncated gene products, or to assess the functions of specific segments of a coding sequence. Noncoding regions can be deleted to identify and characterize regulatory sequences such as promoters. Insertion mutationscan be made by the addition of any desired sequences and may be used to produce fusion proteins. Substitution mutations, which can correspond to one or more nucleotides, are often made in coding or noncoding regions. Included in the former class are nonsense mutations, in which a termination codon is introduced, and missense mutations, in which a single nucleotide or a codon is changed, resulting in the synthesis of a protein with a single amino acid substitution. The introduction of a termination codon is frequently exploited to truncate a membrane protein so that it is secreted or to eliminate the synthesis of a protein without changing the size of the viral genome or mRNA. Substitutions are used to assess the roles of specific nucleotides in regulatory sequences or of amino acids in protein function, such as polymerase activity or binding of a viral protein to a cell receptor.
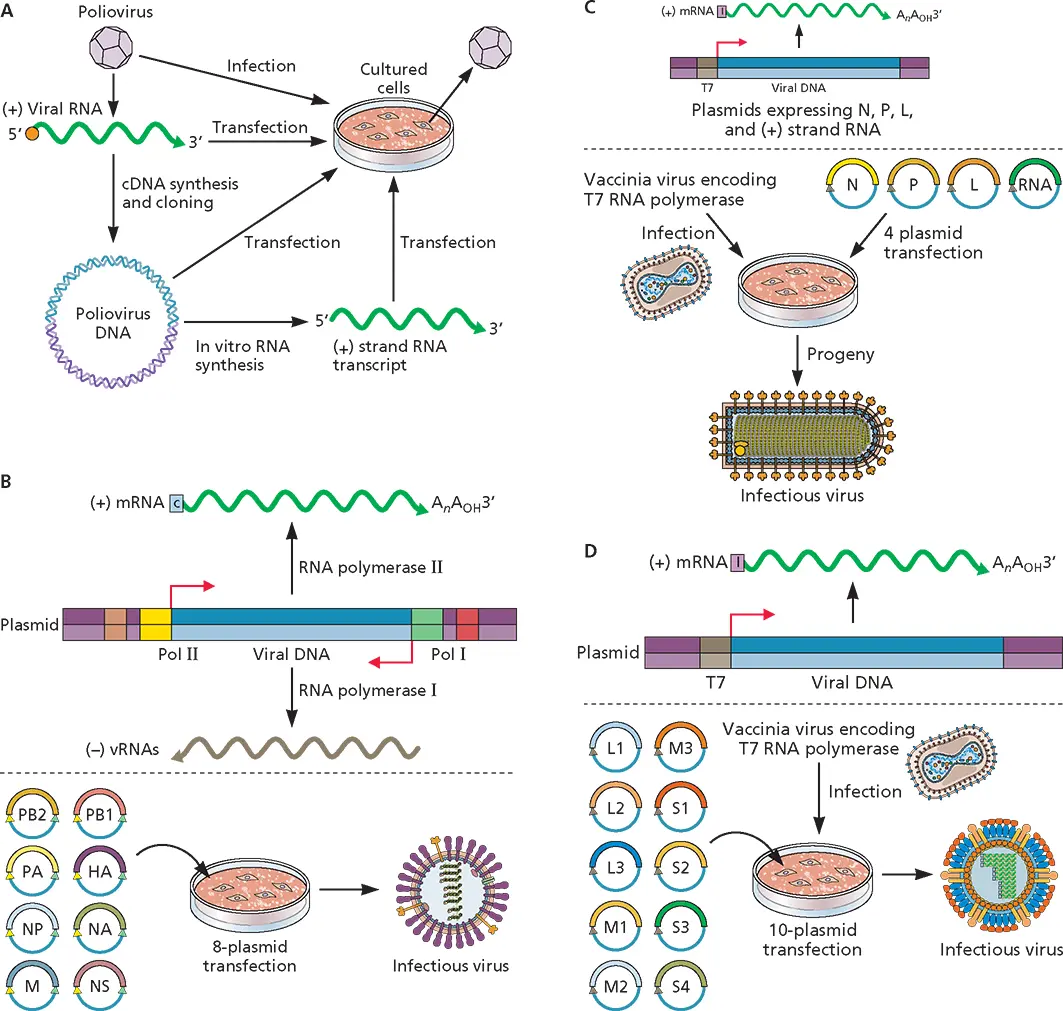
Figure 3.12 Recovery of infectivity from cloned DNA of RNA viruses. (A)The infectivity of cloned DNA of the (+) strand poliovirus RNA genome, which is infectious when introduced into cultured cells by transfection. A complete DNA clone of the viral RNA (blue strands), carried in a plasmid, is also infectious, as are RNAs derived by in vitro transcription of the full-length DNA. (B)Recovery of influenza viruses by transfection of cells with eight plasmids. Cloned DNA of each of the eight influenza virus RNA segments is inserted between an RNA polymerase I promoter (Pol I [green]) and terminator (brown), and an RNA polymerase II promoter (Pol II [yellow]) and a polyadenylation signal (red). When the eight plasmids are introduced into mammalian cells, (–) strand viral RNA (vRNA) molecules are synthesized from the RNA polymerase I promoter, and mRNAs are produced by transcription from the RNA polymerase II promoter. The mRNAs are translated into viral proteins, and infectious virus is produced from the transfected cells. For clarity, only one cloned viral RNA segment is shown. (C)Recovery of infectious virus from cloned DNA of viruses with a (–) strand RNA genome. Cells are infected with a vaccinia virus recombinant that synthesizes T7 RNA polymerase and transformed with plasmids that encode a full-length (+) strand copy of the viral genome RNA and proteins required for viral RNA synthesis (N, P, and L proteins). Production of RNA from these plasmids is under the control of the bacteriophage T7 RNA polymerase promoter (brown). Because bacteriophage T7 RNA transcripts are uncapped, an internal ribosome entry site (I) is included so the mRNAs will be translated. After the plasmids are transfected into cells, the (+) strand RNA is copied into (–) strands, which serve as templates for mRNA synthesis and genome replication. The example shown is for viruses with a single (–) strand RNA genome (e.g., rhabdoviruses and paramyxoviruses). A similar approach has been demonstrated for bunyamwera virus, with a genome comprising three (–) strand RNAs. (D)Recovery of infectious virus from cloned DNA of dsRNA viruses. Cloned DNA of each of the 10 reovirus dsRNA segments is inserted under the control of a bacteriophage T7 RNA polymerase promoter (brown). Because bacteriophage T7 RNA transcripts are uncapped, an internal ribosome entry site (I) is included so the mRNAs will be translated. Cells are infected with a vaccinia virus recombinant that synthesizes T7 RNA polymerase and transformed with all 10 plasmids. For clarity, only one cloned viral RNA segment is shown.
Introducing Mutations into the Viral Genome
Mutations can be introduced into a viral genome when it is cloned in its entirety. Mutagenesis is usually carried out on cloned subfragments, which are then substituted into full-length cloned DNA. This step can now be bypassed by using CRISPR/Cas9 to introduce mutations into complete DNA copies of viral genomes. Viruses are then recovered by introduction of the mutagenized DNA into cultured cells by transfection. This approach has been applied to cloned DNA copies of RNA and DNA viral genomes.
Introduction of mutagenized viral nucleic acid into cultured cells by transfection may have a variety of outcomes, ranging from no effect to a complete block of viral reproduction. Whether the introduced mutation is responsible for an observed phenotype deserves careful scrutiny ( Box 3.10).
Reversion Analysis
The phenotypes caused by mutation can revertin one of two ways: by change of the mutation to the wild-type sequence or by acquisition of a mutation at a second site, either in the same gene or a different gene. Phenotypic reversion caused by second-site mutation is known as suppression, or pseudoreversion, to distinguish it from reversion at the original site of mutation. Reversion has been studied since the beginnings of classical genetic analysis. In the modern era of genetics, cloning and sequencing techniques can be used to demonstrate suppression and to identify the nature of the suppressor mutation (see below). The identification of suppressor mutations is a powerful tool for studying protein-protein and protein-nucleic acid interactions. Some mutations complement changes made at several sites, whereas allele-specificsuppressor mutations complement only a specific change. The allele specificity of second-site mutations provides evidence for physical interactions among proteins and nucleic acids.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Principles of Virology, Volume 1»
Представляем Вашему вниманию похожие книги на «Principles of Virology, Volume 1» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Principles of Virology, Volume 1» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.



