CONTENTS
Flow Cytometric Immunophenotyping
Immunohistochemistry
Interpretation and Limitations of Flow Cytometric Immunophenotyping
Problems and Pitfalls
References
Bibliography
Immunophenotyping is the process by which the pattern of expression of antigens by a population of cells is determined. The presence of a specific antigen is recognised by its binding to a labelled antibody. Antibodies can be present in a polyclonal antiserum that is raised in an animal but more often they are well characterised monoclonal antibodies produced by hybridoma technology; a hybridoma is a clone of cells created by the fusion of an antibody‐producing cell with a mouse myeloma cell. Monoclonal antibodies can be labelled with an enzyme or with a chemical, known as a fluorochrome, that under certain circumstances will fluoresce. Immunophenotyping is carried out primarily by flow cytometry or immunohistochemistry. Flow cytometric immunophenotyping is applicable to cells in peripheral blood, bone marrow, body fluids (pleural, pericardial, ascitic and cerebrospinal fluids) and fine needle aspirates. Immunohistochemistry of relevance to haematological disease is applied particularly to trephine biopsy and lymph node biopsy specimens, but also to biopsy specimens from any other tissues where infiltration by haemopoietic or lymphoid cells is suspected.
Flow Cytometric Immunophenotyping
This technique determines cell size, structure (to some extent) and antigen expression. Cells in suspension are first exposed to a combination of fluorochrome‐labelled monoclonal antibodies (or other lectins or ligands) and then pass in a focused stream through a beam of light generated by a laser. Laser‐generated light is coherent (waves of light are parallel) and monochromatic (single wave length/colour). Large multichannel instruments with multiple lasers are used to identify, count, size and otherwise characterise cells that are hydrodynamically focused and pass in a single file through a narrow orifice in a flow cell. The passing of the cell through a light beam leads to both the scattering of light and the excitation of fluorochromes so that they emit a fluorescence signal. Forward scatter (FSC) of light at a narrow angle is detected and measured and is proportional to cell size. Sideways or side scatter (SSC) of light is detected and measured and is proportional to cell granularity and complexity. Antigens expressed on the surface membrane of cells or, with modified techniques, within cells are detected. After ‘permeabilisation’, both cytoplasmic and nuclear antigens can be detected.
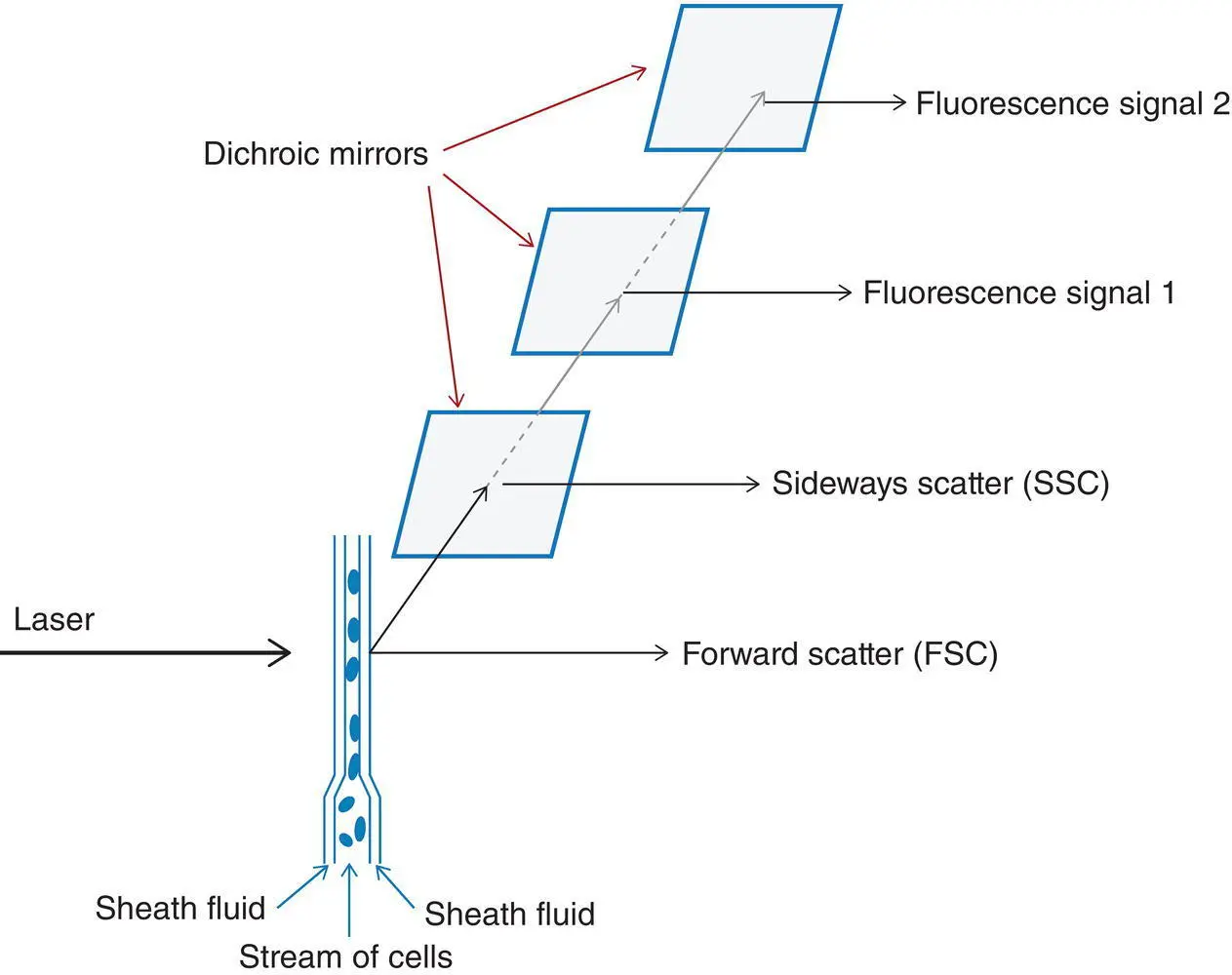
Figure 1.1 Diagrammatic representation of the principles of flow cytometric immunophenotyping.
For each fluorochrome, a selected laser emits light of a specified wavelength that will be absorbed by the fluorochrome. This leads to excitation of the fluorochrome with subsequent emission of light of lower energy and a longer wavelength as the fluorochrome returns to its basal state; this property is known as fluorescence. The amount of light emitted (the number of photons) is proportional to the amount of fluorochrome bound to the cell. The mean fluorescence intensity of a population indicates the strength of expression of the relevant antigen. The emitted light passes through dichroic mirrors, that is, mirrors that reflect some wavelengths and transmit others, so that it is possible, for example, to reflect SSC for measurement and transmit fluorescence signals to another detector such as a photomultiplier tube ( Figure 1.1). The detector produces an electrical signal that is proportional to the amount of incident light. Some commonly used fluorochromes are shown in Table 1.1.
The cells that are studied must be dispersed. For peripheral blood and bone marrow aspirate specimens, it is necessary to exclude mature and immature red cells. This is most simply done by lysing red cells using an ammonium chloride solution. Otherwise red cells and their precursors will appear in scatter plots and interfere with gating leucocyte populations of interest. If assessment of immunoglobulin expression is required, there must also be a washing step to remove the plasma that contains immunoglobulin, which would neutralise the monoclonal lambda‐ or kappa‐specific antibody.
Table 1.1 Commonly used fluorochromes.
| Fluorescein isothiocyanate (FITC) |
| Phycoerythrin (PE) |
| Allophycocyanine (APC) |
| Peridinin chlorophyll (PerCP) |
| Cyanine 5 (Cy5), cyanine 5.5 (Cy5.5) and cyanine 7 (Cy7) |
| Texas red |
| Pacific blue |
| Brilliant violet |
| Krome orange |
| Alexa Fluor 488 (AF488) |
| Alexa Fluor 647 (AF647) |
| Phycoerythrin‐Texas Red X (ECD) |
| Phycoerythrin‐cyanine 5 (PE‐Cy5) |
| Phycoerythrin‐cyanine 5.5 (PE‐Cy5.5) |
| Phycoerythrin‐cyanine 7 (PE‐Cy7) |
The great majority of monoclonal antibodies used in immunophenotyping have been characterised at a series of international workshops and those with the same specificity have been assigned a cluster of differentiation (CD) number. This number can be used to refer to both the antibody and the antigen it recognises. There are now more than 350 specificities recognised so that a careful selection of antibodies for diagnostic use is important. In addition to fluorochromes conjugated to monoclonal or polyclonal antibodies, it is also possible to use either fluorochromes that can bind directly to cellular constituents, such as DNA, or labelled modified aerolysins that bind to membrane glycosylphosphatidylinositol glycan A (GPI) (used in the diagnosis of paroxysmal nocturnal haemoglobinuria). Propidium iodide binding can be used to identify non‐viable cells and exclude them from analysis. Monoclonal antibodies that are most used in flow cytometric immunophenotyping are detailed in Part 2.
Results of immunophenotyping are usually shown as a two‐dimensional plot in which FSC, SSC and the expression of certain antigens are plotted against each other, permitting the recognition of the probable nature of a cell cluster in a particular position. It is thus possible to gate on a cellular population of interest. A gate is an electronic boundary; it can either be predetermined or drawn by the operator. There are four commonly used approaches to gating of target populations: FSC versus SSC, CD45 versus SSC, CD19 versus SSC and CD34 versus SSC.
FSC versus SSC is a useful way of screening a specimen to identify normal populations and to highlight abnormal cells as illustrated in Figure 1.2.
Forward scatter is increased in relation to increasing cell size whilst SSC is influenced by cytoplasmic granularity and nuclear complexity. It is a useful means of gating on blasts when CD34 is not expressed, for example in monoblastic leukaemias. Such plots are helpful in identifying large activated lymphocytes, an excess of small lymphocytes or monocytes and even the presence of hairy cells (see Chapter 3). Granular blasts show increased SSC and this is reflected in a shift to the right in the scatter plot. This can be an early indication of a possible acute promyelocytic leukaemia.
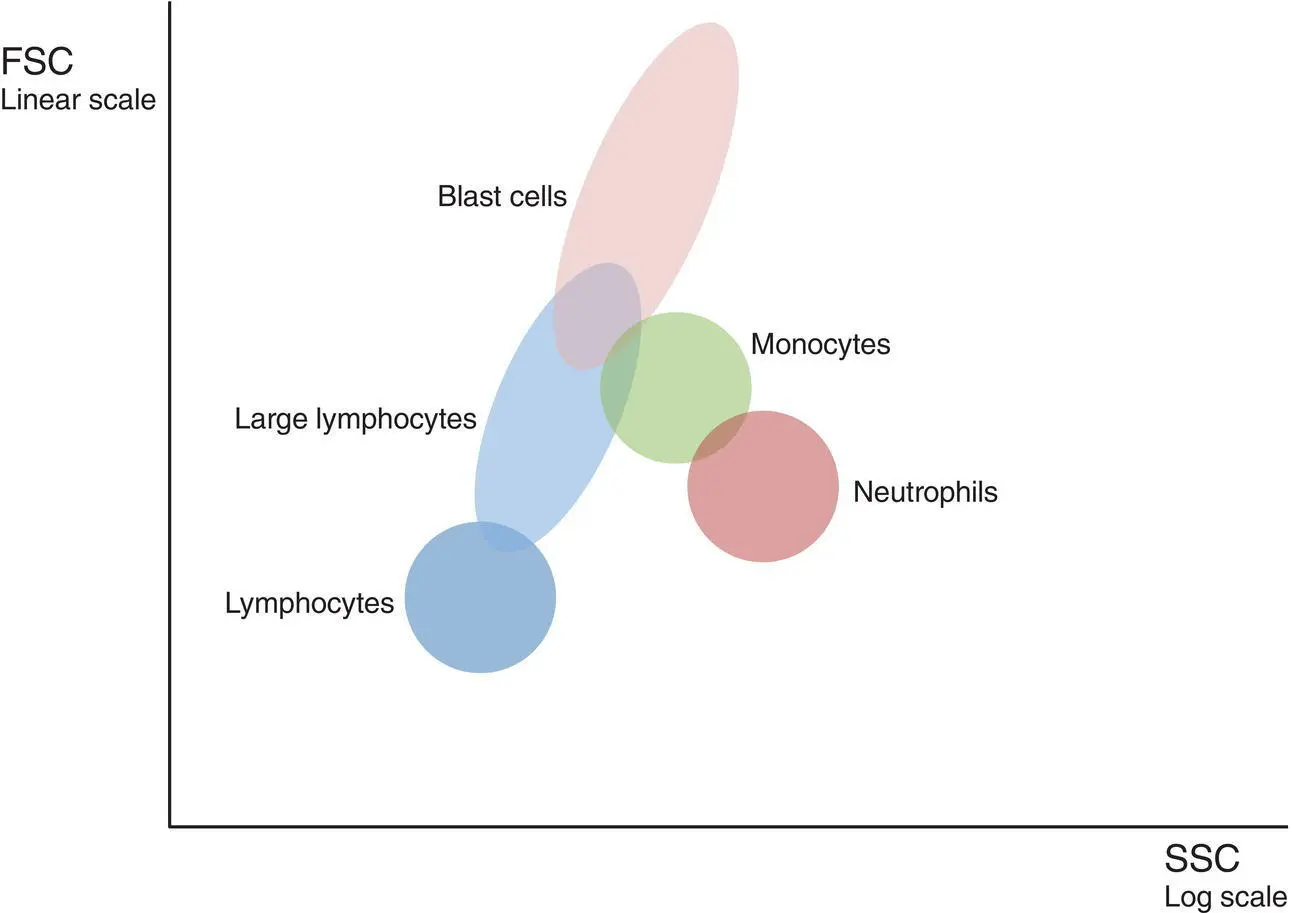
Figure 1.2 Delineation of peripheral blood leucocyte populations using forward scatter (FSC) and side scatter (SSC) characteristics.
Читать дальше














