Preconception carrier screening is being offered for an increasing number of genetic conditions and to a broadening population of patients. It is incumbent on providers to stay abreast of the types of screening offered through clinical and commercial laboratories. We must educate pregnant women and those considering pregnancy on the availability of screening. As screening expands, so must our pretest and post‐test counseling surrounding the potential benefits and risks of this screening and the implications and follow‐up for both positive and negative results. Professional guidelines should continue to be updated to reflect scientific developments and the changing role of carrier screening.
1 American College of Obstetricians and Gynecologists. Carrier screening for genetic conditions. Committee Opinion 691. Obstet Gynecol 2017; 129:e41–55.
2 American College of Obstetricians and Gynecologists. Family history as a risk assessment tool. Committee Opinion 478. Obstet Gynecol 2011; 117:747–50.
3 American College of Obstetricians and Gynecologists. Carrier screening in the age of genomic medicine. Committee Opinion 690. Obstet Gynecol 2017; 129:e35–40.
4 Brennan ML, Schrijver I. Cystic fibrosis: a review of associated phenotypes, use of molecular diagnostic approaches, genetic characteristics, progress, and dilemmas. J Mol Diagn 2016; 18:3–14.
5 Edwards J, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine – points to consider. A joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal‐Fetal Medicine. Obstet Gynecol 2015; 125:653–62.
6 Hoffman JD, Park JJ, Schreiber‐Agus N, et al. The Ashkenazi Jewish carrier screening panel: evolution, status quo, and disparities. Prenat Diagn 2014; 34:1161–7.
7 Hussein N, Weng SF, Kai J, Kleijnen J, Qureshi N. Preconception risk assessment for thalassemia, sickle cell disease, cystic fibrosis, and Tay–Sachs disease. Cochrane Database Syst Rev 2015;( 8):CD010849.
8 Kraft SA, Duenas D, Wilifond BS, Goddard KAB. The evolving landscape of expanded carrier screening: challenges and opportunities. Genet Med 2019; 21:790–7.
9 MacDonald WK, Hamilton D, Kuhle S. SMA carrier testing: a meta‐analysis of differences in test performance by ethnic group. Prenat Diagn 2014; 34:1219–26.
10 Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with permutation or intermediate alleles. Am J Hum Genet 2003; 72:454–64.
11 Vrettou C, Kakourou G, Mamas T, Traeger‐Synodinos J. Prenatal and preimplantation diagnosis of hemoglobinopathies. Int J Lab Hematol 2018; 40:74–82.
PART 3 Maternal Disease
PROTOCOL 11 Maternal Anemia
Elaine Duryea
Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX, USA
A comprehensive review of all causes of anemia may be intimidating, and many available algorithms are not high yield when evaluating pregnant women. The protocol presented here provides an algorithm for the initial evaluation of anemia in pregnancy, with treatment algorithms for the most common causes in pregnancy. Women who do not meet one of the definitions provided below are best managed in consultation with a hematologist.
During a singleton gestation, plasma volume doubles (increasing approximately 1000 mL) and red blood cell (RBC) volume increases by 25% (300 mL), with RBC production lagging slightly behind plasma expansion. This results in a physiological dilutional anemia of pregnancy, which reaches a nadir during the late second to early third trimester ( Table 11.1). The Centers for Disease Control and Prevention (CDC) define anemia as a hemoglobin (Hb) below the fifth centile for a healthy, iron‐supplemented population. This translates to a threshold of Hb less than 11 g/dL in the first and third trimesters, and less than 10.5 g/dL during the second trimester. Rates of anemia in pregnancy vary widely between different populations and socioeconomic classes.
In developed countries, maternal anemia has been associated with increased risk of preterm birth and low‐birthweight infants, as well as neonatal and perinatal death. In addition, maternal complications associated with anemia include preeclampsia, cesarean delivery, postpartum depression, and an increased likelihood for transfusion either intrapartum or postpartum despite equivalent blood loss. Women with anemia are asymptomatic or describe vague symptoms such as fatigue and palpitations; therefore, screening for anemia during pregnancy is recommended regardless of symptoms.
Table 11.1 Changes in laboratory values in pregnancy
Source: Based on ACOG Practice Bulletin No. 107, 2009.
|
Nonpregnant women |
Pregnant women |
| Hemoglobin (g/dL) |
12–16 |
11–14 |
| Hematocrit |
36–46% |
33–44% |
| RBC count (× 10 6/mL) |
4.8 |
4.0 |
| MCV (fL) |
80–100 |
= |
| MCHC |
31–36% |
= |
| Reticulocytes (× 10 9/L) |
50–150 |
= |
| Ferritin (ng/mL) |
>25 |
>20 |
| RDW (red cell distribution width) |
11–15% |
= |
=, unchanged.
Diagnostic work‐up and treatment
Causes of anemia may be classified by pathophysiology, such as comparing acquired versus inherited causes, or mechanisms of anemia such as decreased RBC production versus increased destruction. In clinical practice, more often the evaluation of maternal anemia starts with the mean corpuscular volume (MCV), based on which anemias are defined as microcytic (less than 80 fL), normocytic (80–100 fL) or macrocytic (greater than 100 fL) ( Figure 11.1).
Figure 11.1shows the appropriate work‐up for the search of causes in the presence of macrocytic anemia. Vitamin B 12 deficiency is rare, as most healthy individuals have 2–3 years’ storage available in the liver. However, vitamin B 12deficiency can be encountered in individuals who have undergone bariatric surgery with partial gastric resection and are noncompliant with recommended vitamin B 12supplementation (350 μg/day sublingually plus 1000 μg IM every three months if needed), in individuals with pernicious anemia (an extremely uncommon autoimmune disease in women of reproductive age which is diagnosed by the presence of serum intrinsic factor antibodies), and in those with malabsorption (e.g., Crohn disease or ileal resection). Folate deficiency is less common today given the supplementation of foods with folate and universal folic acid supplementation in pregnancy. Recommended folate requirements are 400 μg/day during pregnancy, with higher doses recommended in the presence of multiple gestations, hemolytic disorders such as sickle cell anemia or thalassemia, and in patients taking antiepileptic drugs (AED) or sulfa drugs (e.g., sulfasalazine). In addition to macrocytic anemia, folate deficiency may also cause thrombocytopenia.
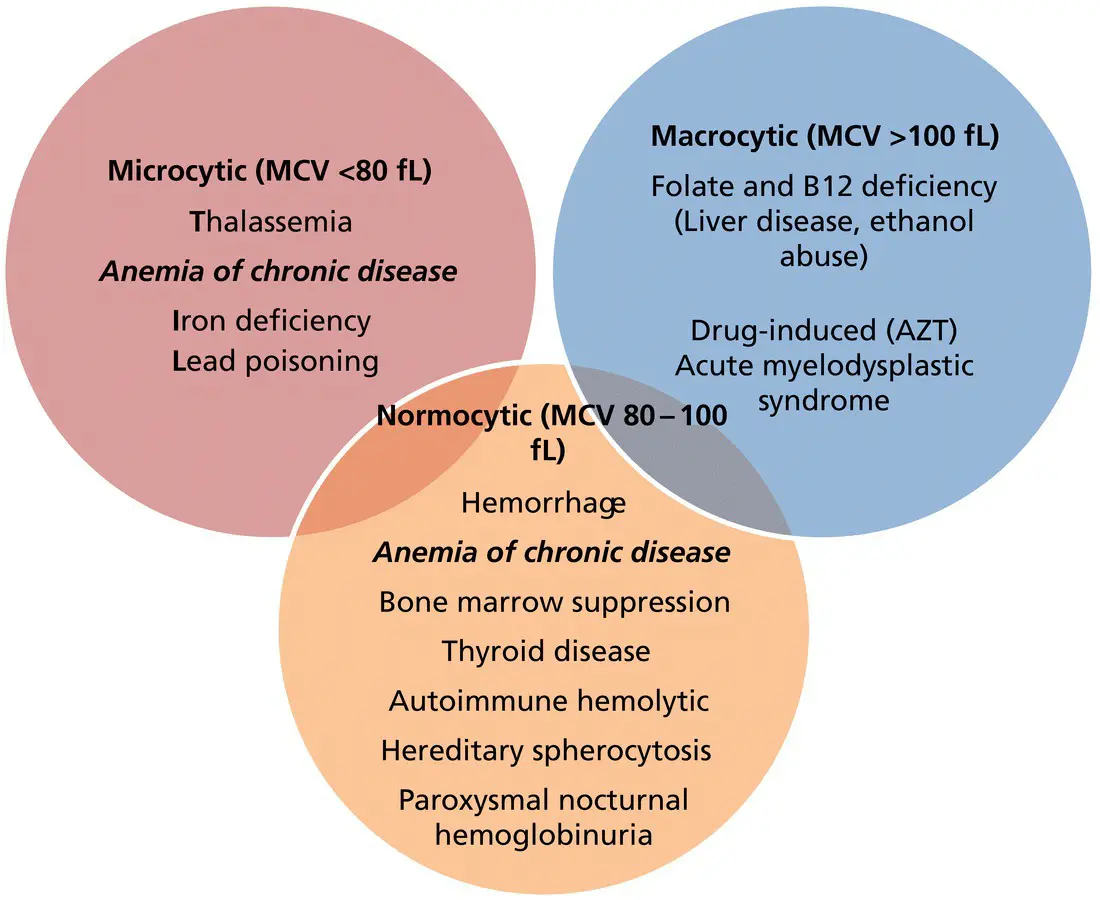
Figure 11.1 Causes of maternal anemia, classified by mean corpuscular volume (MCV).
Читать дальше













