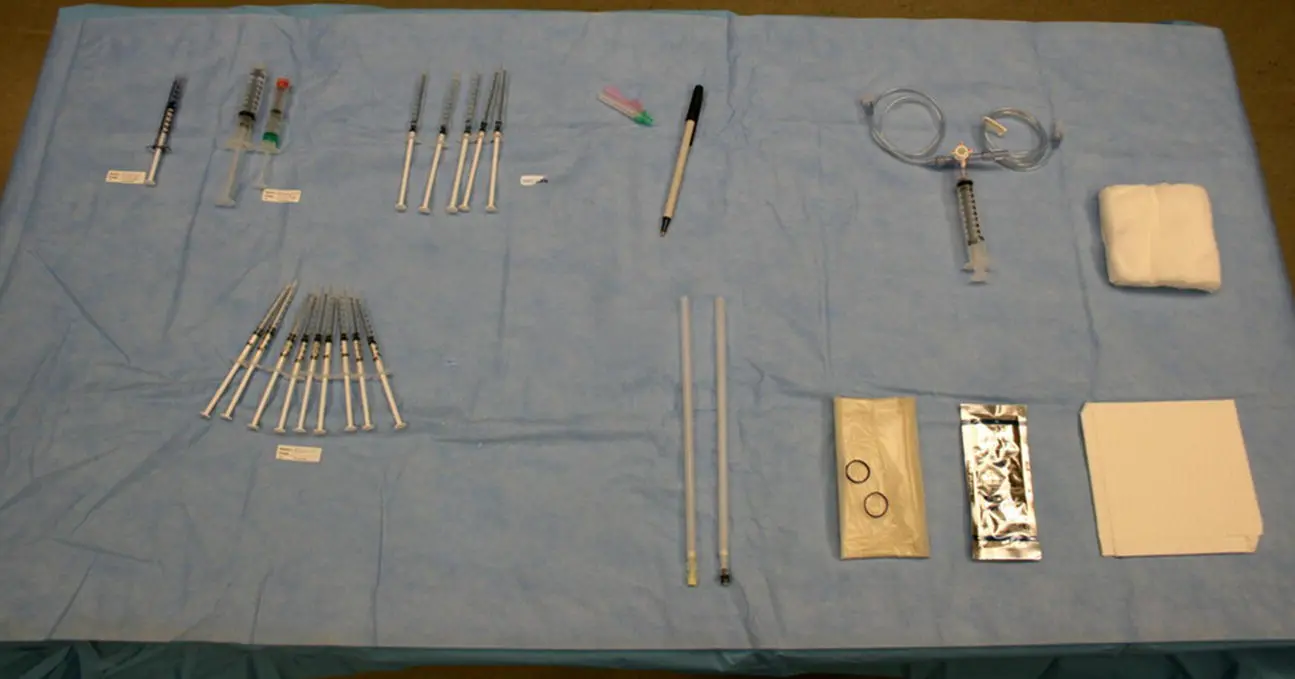
Figure 9.2 A typical OR procedure table set‐up for fetal blood transfusion. Left to right, top : Labeled syringe with fetal dose of fentanyl; labeled syringes with fetal dose of vecuronium; labeled syringes with saline to flush the transfusion circuit as needed; marker; three‐way stopcock with attached 10 or 20 cc syringe in the middle port to serve as blood reservoir, as well as IV tubing, one lateral port leading to the donor blood unit and the other port leading to the fetus; 4 × 4 OR sponges. Left to right, bottom : Syringes with heparin to collect fetal blood samples; 20 or 22 gauge spinal needles of varying length; sterile ultrasound probe cover; sterile ultrasound gel; OR drape.
Accessing the cord is the same as in a diagnostic cordocentesis: using your 22 gauge needle and under continuous ultrasound guidance, you will puncture the fetal umbilical vein and direct the tip of the needle to its lumen. When you think you are in the cord, connect your transfusion circuit to the spinal needle. Maintaining the circuit closed to the donor blood, the middle port of the three‐way stopcock is used to confirm needle placement with a saline flush; you should see turbulent flow within the cord as the fluid is pushed through. Once placement is confirmed, use a heparinized syringe to obtain a fetal blood sample. That initial sample is sent to the OR or STAT lab for processing, and you may start transfusion (at a rate of 3–5 mL/min) while you wait for results.
To transfuse, you will pull blood from the donor unit into the reservoir and then push it towards the fetus from the reservoir, opening and closing the ports of the three‐way stopcock as needed to guide the flow of blood in the right direction. As you transfuse, continue to watch for movement of the blood within the cord, which will appear as echogenic turbulent flow, and keep an eye on the fetal heart rate to make sure the fetus is tolerating the procedure. After you have transfused the necessary blood volume, you will want a closing sample to confirm that you have reached your goal Hct. Once you have removed the needle from the cord, you might observe streaming of blood from the puncture site, which is expected.
The patient post fetal transfusion will need fetal monitoring to ascertain a reassuring fetal status, and to evaluate for preterm labor. There are no data to suggest a particular length of monitoring post procedure, but if contractions are noted on tocometry, a 24‐hour observation period on Labor and Delivery would be reasonable. Most centers perform fetal transfusion up to 35 weeks of gestation with delivery anticipated at 37–38 weeks. However, every fetal transfusion carries the risk of a procedure‐related loss, especially in the already compromised fetus, so every decision for repeat transfusion should be individualized.
Once discharged from the hospital, the patient should return for follow‐up sonographic evaluation within a week. Doppler of the fetal middle cerebral artery and other sonographic findings will still guide timing of next procedure.
The largest volume of cordocenteses and fetal transfusions is done for the evaluation and treatment of fetuses with suspected anemia, usually due to red cell alloimmunization. Besides fetal transfusion, other interventions have been tested to prevent or decrease the severity of fetal anemia in cases of early and severe maternal red cell alloimmunization. These include treatment with either plasmapheresis or IVIG alone or in combination for an attempt at immunomodulation of the disease. There is currently ongoing research into the development of a human IgG1 monoclonal antibody targeting FcRn, the receptor responsible for mediating transfer of IgG across the placenta into the fetal circulation. If these interventions prove successful, they may delay or perhaps eliminate the need for fetal transfusion in some situations.
1 Deka D, Dadhwal V, Sharma AK, et al. Perinatal survival and procedure‐related complications after intrauterine transfusion for red cell alloimmunization. Arch Gynecol Obstet 2016; 293(5):967–73.
2 Dodd JM, Windrim RC, van Kamp I. Techniques of intrauterine fetal transfusion for women with red‐cell alloimmunization for improving health outcomes. Cochrane Database Syst Rev 2012;( 9):CD007096.
3 Hellmund A, Geipel A, Berg C, et al. Early intrauterine transfusion in fetuses with severe anemia caused by parvovirus B19 infection. Fetal Diagn Ther 2018; 43(2):129–37.
4 Lindenburg ITM, Smits‐Wintjens VE, van Klink JM, et al. Long‐term neuro‐developmental outcome after intrauterine transfusion for hemolytic disease of the fetus/newborn: the LOTUS study. Am J Obstet Gynecol 2012; 206:141.e1–148.e8.
5 Lindenburg ITM, van Kamp I, Oepkes D. Intrauterine blood transfusion: current indications and associated risks. Fetal Diagn Ther 2014; 36:263–71.
6 Ruma MS, Moise KJ Jr, Kim E, et al. Combined plasmapheresis and intravenous immune globulin for the treatment of severe maternal red cell alloimmunization. Am J Obstet Gynecol 2007; 196;138.e1–138.e6.
7 Wallace AH, Dalziel SR, Cowan BR, et al. Long‐term cardiovascular outcome following fetal anaemia and intrauterine transfusion: a cohort study. Arch Dis Child 2017; 102(1):40–5.
8 Zwiers C, Lindenburg ITM, Klumper FJ, et al. Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound Obstet Gynecol 2017; 50:180–6.
9 Zwiers C, van der Bom JG, van Kamp I, et al. Postponing Early Intrauterine Transfusion with Intravenous immunoglobulin Treatment: the PETIT study on severe hemolytic disease of the fetus and newborn. Am J Obstet Gynecol 2018; 219:291.e1–9.
10 Zwiers C, van Kamp I, Oepkes D, Lopriore E. Intrauterine transfusion and non‐invasive treatment options for hemolytic disease of the fetus and newborn – review on current management and outcome. Exp Rev Hematol 2017; 10(4):337–44.
PROTOCOL 10 Preconception Genetic Screening
Lauren Sayres1 and Jeffrey A. Kuller2
1Division of Maternal Fetal Medicine, University of Colorado, Aurora, CO, USA
2Department of Obstetrics and Gynecology, Division of Maternal‐Fetal Medicine, Duke University School of Medicine, Durham, NC, USA
Carrier screening is defined as testing performed on individuals to assess whether they carry one allele for a genetic condition for which they do not have the phenotype. The American College of Obstetricians and Gynecologists (ACOG) recommends that all pregnant women be provided information about carrier screening. Ideally, this carrier screening is performed prior to conception to maximize reproductive options and facilitate decision making. Ethnic, panethnic, and expanded carrier screening are all acceptable strategies for carrier screening. ACOG recommends that each obstetrician‐gynecologist or healthcare provider should establish a standard approach to screening. Historically, screening has been offered based on the individual’s pretest risk of carrier status as determined by her racial or ethnic background or her family history. However, in an increasingly multiethnic society, such targeted strategies are being supplanted by panethnic screening algorithms.
With the advent of lower cost, high‐throughput genotyping technology, expanded carrier screening panels that evaluate for hundreds of conditions are being introduced. ACOG has recommended the following screening criteria for disorders that should be included in expanded panels. Carrier frequency should be 1 in 100 or greater, corresponding to a disease incidence of 1 in 40 000. The phenotype associated with the condition should be well defined, occur early in life, and cause significant physical or cognitive impairment or affect quality of life. The condition will ideally be amenable to prenatal diagnosis, changes to antenatal or delivery management, and parental education about coordination of care after delivery. In contrast to diseases for which newborn screening is mandated, carrier screening is offered for lethal conditions such as Tay–Sachs disease that do not necessarily have postnatal intervention strategies.
Читать дальше













