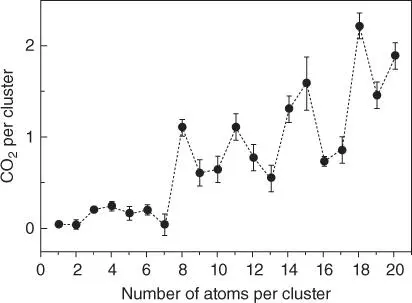
Figure 5.3 Size‐dependent overall CO oxidation reactivity of gold clusters, Au n, supported on defect‐rich MgO(100) films, expressed as the number of CO 2molecules per cluster.
Source: Reprinted with permission from Sanchez et al. [8]. Copyright 1999, American Chemical Society.
Strongly size‐dependent properties and reactivity of metal clusters inspired motto of the cluster science field – each atom counts! However, this UHV cluster source methodology is limited to the deposition onto flat supports (contrary to the powders of porous materials with substantial internal surface area that are often used in heterogeneous catalysis). Such UHV methodology is also not ideal with respect to the overall atomic efficiency of the process because a mixture of clusters with a wide range of sizes is produced while only one type of cluster with specific size is mass‐filtered to be deposited onto the support, with the remaining clusters effectively wasted. Furthermore, relatively low quantities of size‐filtered clusters limit the potential for scale‐up (10 13clusters in the example above is 10 orders of magnitude lower than 1 mol of clusters). However, this method truly stands out in the fabrication of model heterogeneous catalysts for studies of catalytic processes involving the delivery of reagents from the gas phase since the instruments for catalyst fabrication can be relatively easily adapted to perform model catalytic experiments without exposure of the freshly made catalyst to air (avoiding contamination with adsorbed water, adventitious hydrocarbons, etc.).
Several recent detailed review papers are available for the curious reader interested in specific details of catalytic studies that utilize materials made using UHV size‐selected cluster deposition [4, 9, 10]. Highlights of several milestone papers will be briefly given in the following section. Pt group metals are known to catalyze CO oxidation – an important pollution cleanup reaction in automotive industry. Not surprisingly, one of the early papers demonstrated a strong dependency of CO oxidation on the size of Pt nclusters ( n = 5–20) on MgO films. An abrupt increase in the activity per Pt atom could be measured from Pt 14(which had similar activity to smaller clusters) to Pt 15, beyond which the activity decreases [11]. With the catalysis by Au NPs capturing the limelight, a detailed investigation of the reactivity of Au nclusters on MgO quickly followed [8]. The authors demonstrated that Au 8is the smallest Au cluster active for CO oxidation at temperatures as low as 140 K! Follow‐up studies elucidated several key factors including “the role of the metal‐oxide support and its defects, the charge state of the cluster, structural fluxionality of the clusters, electronic size effects, the effect of an underlying metal support on the dimensionality, charging and chemical reactivity of gold nanoclusters adsorbed on the metal‐supported metal‐oxide, and the promotional effect of water” [12, 13]. Fluxionality of even macroscopic ideally flat metal surfaces can be manifested by restructuring in the presence of CO, such as in the case of Pt(110) [14]. However, fluxionality is much greater in the case of ultrasmall metal clusters, which, in part, explains their uniquely high reactivity. Fluxionality of supported clusters is not confined to Au clusters – higher activity of Pt 7(cf. that of Pt 4and Pt 8in catalytic dehydrogenation of ethylene at higher temperatures was also attributed to fluxionality because this cluster can transform to a single‐layer isomer) [15].
More recent examples of superior catalytic activity of catalysts made under UHV using size‐selected clusters deposited onto supports include:
1 (a) elucidation of Pt cluster size effects in photocatalytic hydrogen production from water, demonstrating superior activity of Pt46‐based catalyst [16];
2 (b) Pt cluster size effects and the effect of particle proximity in the oxygen reduction reaction, which is important in fuel cells [17];
3 (c) proof that Pd6 and Pd17 clusters deposited on nanocrystalline diamond are among the most active (in terms of turnover rate per Pd atom) catalysts known for the oxygen evolution reaction, which is currently the bottleneck in electrocatalytic water splitting to H2 and O2 [18];
4 (d) demonstration that Cu4 clusters on Al2O3 are the most active in CO2 hydrogenation to methanol under low pressure and temperature [19].
Even bimetallic clusters can be made using UHV cluster deposition techniques utilizing a dual‐target magnetron sputtering system and mixing of the plume of growing clusters in the aggregation chamber. A very recent study of Ag–Pt clusters deposited on Al 2O 3highlights the importance of unambiguous selection and use of mass spectrometry to screen clusters present in the aggregation chamber, where Ag 9Pt 2 +and Ag 9Pt 3 +clusters were selected without overlap with any other species. The catalysts so obtained had superior activity and stability in CO oxidation [20].
5.3 Chemically Synthesized Metal Clusters
The founding father of cluster chemistry, Professor Alfred Cotton, defined clusters as “those containing a finite group of metal atoms which are held together entirely, mainly, or at least to a significant extent, by bonds directly between the metal atoms even though some non‐metal atoms may be associated intimately with the cluster” [21]. He continued: “This is essentially the definition suggested earlier [22], but broadened to include compounds in which the metal atoms are held together entirely by metal–metal bonds. It is broad enough also to include compounds containing only two metal atoms, although these are atypic in the same sense as methane is an atypic aliphatic hydrocarbon. It also includes clusters in which not all the metal atoms are identical, although at present scarcely any such clusters, except for binuclear ones, have been identified.” The history of the early developments in the field is nicely covered by Cotton in the review he published later in his career [23].
In addition to cluster cores containing several metal atoms connected by direct bonds established during chemical synthesis, clusters contain ligands (from Latin ligandus , gerund of ligãre , meaning “to bind”): ions or small molecules bonded to the metal atoms via donor atom within the ligand.
Although this field started with clusters containing O and Cl ligands bonded to the metal cluster core, the field soon exploded with advances in cluster chemistry using CO (metal carbonyl clusters) [24–26], phosphines (PR 3) [27] and chalcogen‐based ligands (e.g. ligands containing S, Se, or Te as a donor atom) [28], and, in particular, thiols (‐SR) [29]. Other common organometallic ligands include alkynes [30] and aromatic cyclopentadienyl (C 5R 5 −) or arene (C 6R 6) ligands, which can bind to the metal cluster core via all five or six carbon atoms, respectively [31, 32]. Transition metals or transition metals with main‐group elements [33] as well as pure main‐group elements [34] and even lanthanides [35] can form clusters.
Chemically made metal clusters can be made in a variety of ways, from “one‐pot” synthesis to multistep sequences of reactions. For example, structurally similar undecagold (Au 11(PPh 3) 7Cl 3or “Au 11–7” and [Au 11(PPh 3) 8Cl 2]Cl or “Au 11–8”) clusters stabilized by phosphine ligands can be synthesized using “one‐pot” synthesis approaches by reducing the same mononuclear precursor Au(PPh 3)Cl, with sub‐stoichiometric (0.25 molar equivalents) amounts of NaBH 4favoring Au 11–8, while excess (5 molar equivalents) yield Au 11–7[27]. In contrast, the synthesis of [PtRu 5C(CO) 15(μ‐SnPh 2)(μ 6‐C)] starting from RuCl 3involves seven steps, and, although product yields at each individual step are quite high, the overall yield is low, and synthesis process is quite protracted [36]. Each specific subclass of chemically synthesized cluster species has got specific methodologies for building up cluster core, such as thermolysis of metal carbonyl clusters [24] or sequential reduction in gold‐thiolate clusters, as well as various periphery core atom substitution/addition reactions and even “etching” of less stable clusters in the mixture for size “focusing” to the most stable cluster out of the range present in the initial mixture [29]. Despite the maturity of the field, there is no single overarching synthesis methodology that could guarantee access to chemically made clusters of every possible nuclearity (number of metal atoms in the cluster core) and composition (for mixed metal clusters 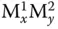 , some, but not necessarily all, combinations of x and y can be made). However, significant progress has been made toward rational total synthesis of thiolate‐protected metal nanoclusters, as highlighted in a recent review [29].
, some, but not necessarily all, combinations of x and y can be made). However, significant progress has been made toward rational total synthesis of thiolate‐protected metal nanoclusters, as highlighted in a recent review [29].
Читать дальше


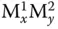 , some, but not necessarily all, combinations of x and y can be made). However, significant progress has been made toward rational total synthesis of thiolate‐protected metal nanoclusters, as highlighted in a recent review [29].
, some, but not necessarily all, combinations of x and y can be made). However, significant progress has been made toward rational total synthesis of thiolate‐protected metal nanoclusters, as highlighted in a recent review [29].