19 19 Sahoo, P.K., Aepuru, R., Panda, H.S., and Bahadur, D. (2015). Sci. Rep. 5: 17726.
20 20 Jiafu, Q., Dongyun, C., Najun, L. et al. (2018). Small 14: 1800343.
21 21 Zhao, Y., Xie, X., Zhang, J. et al. (2015). Chem. Eur. J. 21: 15908–15913.
22 22 Yin, X.‐P., Wang, H.‐J., Tang, S.‐F. et al. (2018). Angew. Chem. Int. Ed. 57: 9382–9386.
23 23 Mochalin, V.N., Shenderova, O., Ho, D., and Gogotsi, Y. (2011). Nat. Nanotechnol. 7: 11.
24 24 Zeiger, M., Jäckel, N., Mochalin, V.N., and Presser, V. (2016). J. Mater. Chem. A 4: 3172–3196.
25 25 Liu, J., Yue, Y., Liu, H. et al. (2017). ACS Catal. 7: 3349–3355.
26 26 Roldán, L., Benito, A.M., and García‐Bordejé, E. (2015). J. Mater. Chem. A 3: 24379–24388.
27 27 Tian, S., Fu, Q., Chen, W. et al. (2018). Nat. Commun. 9: 2353.
28 28 Bavykina, A.V., Goesten, M.G., Kapteijn, F. et al. (2015). ChemSusChem 8: 809–812.
29 29 Beine, A.K., Kruger, A.J.D., Artz, J. et al. (2018). Green Chem. 20: 1316–1322.
30 30 Soorholtz, M., Jones, L.C., Samuelis, D. et al. (2016). ACS Catal. 6: 2332–2340.
31 31 Jens, A., Sabrina, M., and Regina, P. (2015). ChemSusChem 8: 672–679.
32 32 Bavykina, A.V., Rozhko, E., Goesten, M.G. et al. (2016). ChemCatChem 8: 2217–2221.
33 33 Kamiya, K., Kamai, R., Hashimoto, K., and Nakanishi, S. (2014). Nat. Commun. 5: 5040.
34 34 Yamaguchi, S., Kamiya, K., Hashimoto, K., and Nakanishi, S. (2017). Chem. Commun. 53: 10437–10440.
35 35 Ryo, K., Kazuhide, K., Kazuhito, H., and Shuji, N. (2016). Angew. Chem. Int. Ed. 55: 13184–13188.
36 36 Titirici, M.‐M. and Antonietti, M. (2010). Chem. Soc. Rev. 39: 103–116.
37 37 Makowski, P., Demir Cakan, R., Antonietti, M. et al. (2008). Chem. Commun. https://doi.org/10.1039/B717928F.
38 38 Titirici, M.‐M., Antonietti, M., and Thomas, A. (2006). Chem. Mater. 18: 3808–3812.
39 39 Xiong, H., Pham, H.N., and Datye, A.K. (2013). J. Catal. 302: 93–100.
40 40 Graham, U.M., Jacobs, G., Gnanamani, M.K. et al. (2014). ACS Catal. 4: 1662–1672.
41 41 den Breejen, J.P., Sietsma, J.R.A., Friedrich, H. et al. (2010). J. Catal. 270: 146–152.
42 42 Casavola, M., Hermannsdörfer, J., de Jonge, N. et al. (2015). Adv. Funct. Mater. 25: 5309–5319.
43 43 Arrigo, R., Schuster, M.E., Xie, Z. et al. (2015). ACS Catal. 5: 2740–2753.
44 44 Pylypenko, S., Borisevich, A., More, K.L. et al. (2013). Energy Environ. Sci. 6: 2957–2964.
45 45 Vriamont, C., Haynes, T., McCague‐Murphy, E. et al. (2015). J. Catal. 329: 389–400.
46 46 Sun, S., Zhang, G., Gauquelin, N. et al. (2013). Sci. Rep. 3: 1775.
47 47 Xiao, M., Zhu, J., Ma, L. et al. (2018). ACS Catal. https://doi.org/10.1021/acscatal.8b00138.
48 48 Lin, Y., Watson, K.A., Ghose, S. et al. (2009). J. Phys. Chem. C 113: 14858–14862.
49 49 Deng, D., Chen, X., Yu, L. et al. (2015). Sci. Adv. 1 (11): e1500462.
50 50 Casaban, J., Hardacre, C., James, S.L., and Lagunas, M.C. (2012). Green Chem. 14: 1643–1648.
51 51 Konda, S.K., Amiri, M., and Chen, A. (2016). J. Phys. Chem. C 120: 14467–14473.
52 52 Almkhelfe, H., Li, X., Thapa, P. et al. (2018). J. Catal. 361: 278–289.
5 Metal Cluster‐Based Catalysts
Vladimir B. Golovko
School of Physical and Chemical Sciences, University of Canterbury, Christchurch, New Zealand
A vast majority (∼85–90%) of synthesis processes used by chemical industry involve catalysts, with solid or heterogeneous catalysts utilized in up to ∼80–85% of catalytic processes [1]. One of the major advantages of heterogeneous catalysts is the ease of catalyst recovery, recycling, and, ultimately, the possibility of continuous flow through operation of reactors. Metal‐based catalysts employed by industry typically contain metal nanoparticles (NPs) dispersed on various supports. The prefix “nano‐” (from Greek “dwarf”) stands for 10 −9; thus, NPs have sizes measured in nanometers or 10 −9m. As Professor Ertl aptly pointed in his 2007 Nobel Prize in Chemistry talk, “In fact catalysis has been a nanotechnology long before this term was introduced”. The atomic efficiency of metal utilization is vastly improved by dividing metal into NPs, which have extremely high surface‐to‐volume ratios (minimizing amount of metal buried inside particles), resulting in the high proportion of atoms at, or very close to, the surface (where catalytic action takes place). However, industrially used large‐scale and cost‐efficient catalyst fabrication methods typically do not allow solid catalysts to be made with precisely defined active sites. A range of particle sizes is typically present in materials made using industrial approaches such as coprecipitation. This makes it hard to reliably establish structure–property relationships with respect to the activity and selectivity of the catalysts. Classical surface science research studies using ideally flat surfaces of macroscopic metal crystals were important for developing methodologies for studying solid catalysts, as well as for understanding mechanisms of reactions on such surfaces. However, such studies are intrinsically limited when it comes to grasping effects arising due to the nanosize of a catalytically active particle and effects of its interaction with support. This chapter will introduce the advanced fabrication of model heterogeneous catalysts using atomically precise metal clusters as precursors for well‐defined active sites.
Clusters – what are these species? The term cluster is used over a huge range of sizes – from a cluster of stars, “a group of stars that are gravitationally bound for some length of time,” to a metal cluster, a particle containing specific number of metal atoms chemically bound to each other . In principle, metal‐containing chemical clusters can have wider range of compositions and can include other elements, such as sulfur (metal‐sulfide clusters) or oxygen (metal oxide clusters), within the core; yet, this chapter will focus on pure metal clusters.
Metal clusters occupy a niche between individual metal atoms or ions and larger, bulk‐like metallic NPs. Their sizes range from just a few atoms to more than 100 atoms (typically, sub‐3 nm in size). Metal clusters have strongly size‐dependent electronic properties that can be qualitatively understood in terms of an elementary introduction to band theory (see Figure 5.1). Electron energy levels of a single atom of a particular element are precisely defined and depend on the position of the element in the periodic table, as shown, for example, of an atomic orbital (AO) for an atom (M 1) in Figure 5.1. The formation of a dimer (M 2) yields two molecular orbitals (MOs): The bonding one has lower energy than the AO and, when occupied by electrons, stabilizes the dimer. The empty antibonding orbital has higher energy than the AO, by about the same difference as the bonding MO is stabilized. Adding another atom to create M 3results in the appearance of the nonbonding MO at an energy similar to that of the AO. Progressive growth of the cluster size by additional atoms adds extra MOs and electrons to the system, altering energies of other MOs.
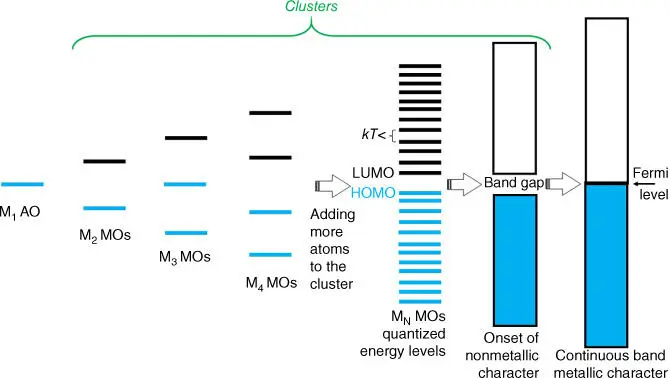
Figure 5.1 Illustration of building up of the band in the bulk metal starting from an individual atom highlighting size sensitivity of electron energy levels in clusters and showing the upper limit of cluster sizes corresponding to size at which transition from semiconducting to metallic behavior occurs. Blue color highlights occupied by electron(s) orbitals. (See online version for color figure).
Читать дальше


