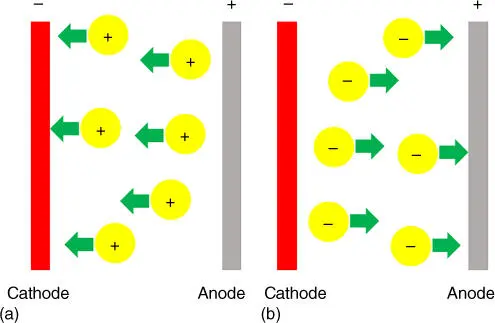
Figure 3.6 Schematic illustration of electrophoretic deposition process: (a) cathodic electrophoretic deposition and (b) anodic electrophoretic deposition.
Unlike electrodeposition, the electrophoretic deposition does not require the liquid medium to be conductance, and in fact organic solvent is a preferred medium. Water is not an ideal medium in electrophoretic deposition because the applied voltage may cause the water splitting reaction that produces hydrogen and oxygen gases at the cathode and anode, respectively. The rigorous evolution of gases could adversely affect the formation of deposit on the electrodes. Because the deposition is greatly determined by the surface charge of particles, this technique offers versatility in depositing complex compounds and composites as long as surface charge can be modulated. One potential weakness, however, is the absence of particle and electrode reactions in electrophoretic deposition. The deposited materials do not lose their charge upon deposition, and therefore a reversal of electric field can result in delamination of the deposited layer. It is important to carefully select similarly charged particles and similar solvent–binder–dispersant systems for having good control over layer thickness.
As the mechanism of electrophoretic deposition involves movement of charged particles in liquid suspension driven by an applied voltage, the important parameters governing the deposition process are therefore related to the regulation of suspension and the physical parameters of the process (e.g. voltage, deposition time, etc.). For the suspension containing the targeted particles, many physicochemical natures of both particle and the liquid medium must be considered. Apparently, particle size suitable for electrophoretic deposition is an important factor. Although there is no general rule of thumb to specify ideal particle size for electrophoretic deposition, it is crucial to have a high dispersion and good colloidal stability to ensure even deposition. Colloidal particles with size smaller than 1 μm in diameter tend to remain in suspension stably owing to the Brownian motion. Therefore, in general, it is the main problem for larger particles as they tend to settle by gravity over shorter period. When the sedimentation of particles occur faster than the electrophoresis‐induced particles mobility, uniform deposition is unlikely: thinner coating on the top region while thicker deposit at the bottom of the substrate. However, this does not necessarily mean that large particles could not be deposited. Colloidal stability of large particle can be improved by having a very strong surface charge, and the electrophoresis movement can be increased with larger electrical double layer region.
Selection of liquid medium is another factor governing the quality of electrophoretic deposition. Dielectric constant of the liquid medium is highly correlated with the deposition performance. Deposition fails in the liquid medium with too low dielectric constant because of weak dissociative power, while electrophoretic mobility is significantly reduced when a medium with high dielectric constant is used due to the reduced size of double layer region. Consequently, liquids of low dielectric constant (but not too low) are a favorable condition. As mentioned earlier, stabilization of suspension, i.e. colloidal stability, is critical in facilitating smooth deposition, and this stability can be achieved by having high and uniform surface charge on the particle. The ζ potential of particle becomes one of the key factors in the electrophoretic deposition. The magnitude of the ζ potential determines the stability of the suspension (repulsive interaction between particles), while the polarity of the ζ potential (positively or negatively charge) decides the direction and migration velocity of the particles during electrophoretic process. The probability of coagulation of the suspended particle must be minimized by modulating the two forces with opposite effects: electrostatic and van der Waals forces. High electrostatic repulsion attributed to the high ζ potential (high particle charge) is desired to suppress agglomeration. Conveniently, the ζ potential can be tuned by carefully selecting pH and additives to the suspension.
3.2.4 Combinatory Methods Involving Electrochemical Process
Although the electrochemical principles discussed in Section 3.2are sufficient to guide a variety of thin film synthesis, recently there are more examples adopting a combinatory approach to prepare new functional thin films. The combinatory approaches can integrate two electrochemical methods or combine an electrochemical method with other technique to synthesize thin film with unique properties. As discussed in the earlier section, anodization is an effective method in producing nanostructured thin films made of simple metal oxide. A wide variety of simple metal oxides such as Al 2O 3, TiO 2, WO 3, SiO 2, MoO 3, and NiO fabricated by anodization process has been reported. Relatively rare is the fabrication of ternary or complex oxide using anodization. Composites of metal oxide decorated with other functional materials are also generally not being reported using anodization process alone. In this section, the design of metal oxide‐based composite and the preparation of ternary oxide thin film using a combinatory electrochemical method will be discussed.
3.2.4.1 Combined Electrophoretic Deposition–Anodization (CEPDA) Approach
Combined electrophoretic deposition–anodization (CEPDA) is a combined electrochemical process that developed to allow the simultaneous anodic growth of metal oxide and the electrophoretic‐driven deposition of reduced graphene oxide (RGO) on the resulting anodic oxide [18]. Metallic foil is employed as the anode in a typical anodization setup as shown in Figure 3.7. Formation of TiO 2nanotube (TNT) arrays following the typical anodization route is expected. However, the organic electrolyte of this anodizing setup is suspended with particles/flakes of RGO with negative surface charge. Within the anodization duration, electrophoretic forces drive the negatively charged RGO to the TiO 2anode to form TiO 2–RGO composite that find uses in photocatalytic reactions. The RGO deposited on TNTs using CEPDA method has a stronger physical interaction between host and guest compared with the typical coating techniques such as spin, drop, and dip coating. This is a demonstration of preparing composite materials by combining two electrochemical working principles in a single experimental process. Otherwise, this kind of composite thin film can be prepared by multistep process. In this combinatory method, both the growth of oxide and its integration with secondary component can take place simultaneously.
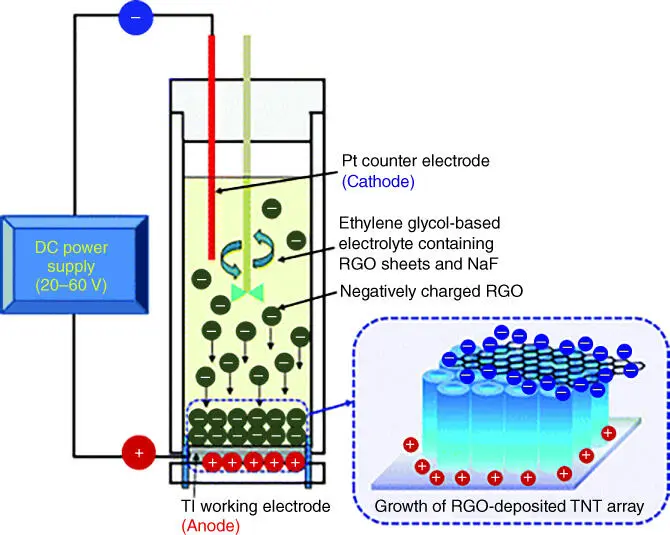
Figure 3.7 Schematic illustration of the apparatus for combined electrophoretic deposition–anodization (CEPDA) approach in the preparation of TiO 2nanotube (TNT)–reduced graphene oxide (RGO) composite.
Source: Yun et al. 2012 [18]. Reproduced with permission of Royal Society of Chemistry.
(See online version for color figure).
Although anodization is, in general, limited to the synthesis of simple oxide thin film, by combining the as‐anodized thin film (before rigid crystallization) with hydrothermal/solvothermal treatment, transformation of the simple metal oxide into complex ternary oxide is possible [18, 33, 34]. Depending on the metallic substrate, bare anodization process usually yields the metal oxide with amorphous phase or partially crystalline structure. Subsequent annealing at elevated temperature is needed to transform the as‐anodized films into well‐crystallized metal oxide that find applications in various reactions. This intermediate state of as‐anodized film offers the possibility to further engineering their composition for final product. For example, upon anodization of tungsten foil, the formed layer is hydrated WO 3(WO 3·2H 2O) with loosely packed structure. Subjecting this as‐anodized WO 3·2H 2O film into a hydrothermal treatment containing bismuth species (Bi 2O 2) 2+forces the interstitial water molecules to be replaced by (Bi 2O 2) 2+. Crystallization process takes place during the hydrothermal treatment, and bismuth tungstate (Bi 2WO 6) thin film is obtained. With proper control, the rate and the depth for the insertion of Bi cations into host material (example is WO 3) can be tuned. As a result, catalytic thin film with two Bi as dopant is made. More interestingly, the concentration of dopant (not limited to Bi) indicates gradient across interfaces. This gradient concentration has important and constructive impact on charge transfer. The combination of anodization with hydrothermal treatment has been proven applicable to prepare different types of ternary oxide films. However, detailed procedures should be strictly considered in this combinatory method. The successful transformation of simple metal oxide into ternary or complex metal oxide is based on the relatively amorphous nature of the as‐anodized metal oxide film. Such transformation is not likely when the highly rigid or crystalline anodized films are employed due to the limited rearrangement of the lattice structure.
Читать дальше



