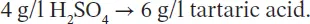1 ...6 7 8 10 11 12 ...41 Consequently, the description of the different steps of enology does not always obey logic as precise as the titles of these works may lead to believe. For example, microbial contamination during aging and storage is covered in Volume 1. The antiseptic properties of SO 2incited the description of its use in the same volume. This line of reasoning led to the description of the antioxidant‐related chemical properties of this compound in the same chapter as well as an explanation of adjuvants to sulfur dioxide: sorbic acid (antiseptic) and ascorbic acid (antioxidant). In addition, the on lees aging of white wines and the resulting chemical transformations cannot be separated from vinification and are therefore also covered in Volume 1. Finally, our understanding of phenolic compounds in red wine is based on complex chemistry. All aspects related to the nature of the corresponding substances, their properties, and their evolution during grape maturation, vinification, and aging are therefore covered in Volume 2.
These works only discuss the principles of equipment used for various enological operations and their effect on product quality. For example, temperature control systems, destemmers, crushers, and presses, as well as filters, inverse osmosis, machines, and ion exchangers are not described in detail. Bottling is not addressed at all. An in‐depth description of enological equipment would merit a detailed work dedicated to the subject.
Wine tasting, another essential role of the winemaker, is not addressed in these works. Many related publications are, however, readily available. Finally, wine analysis is an essential tool that a winemaker should master. It is, however, not covered in these works except in a few particular cases, i.e. phenolic compounds, whose different families are often defined by analytical criteria.
The authors thank the following people who have contributed to the creation of this work:
J.F. Casas Lucas, Chapter 14, Sherry; A. Brugirard, Chapter 14, Sweet Wines; J.N. de Almeida, Chapter 14, Port Wines; A. Maujean, Chapter 14, Champagne; C. Poupot for the preparation of material in Chapters 1, 2, and 13; Miss F. Luye‐Tanet for her help with typing.
They also thank Madame B. Masclef in particular for her important part in the typing, preparation, and revision of the final manuscript.
Pascal Ribéreau‐Gayon
Bordeaux
REMARKS CONCERNING THE EXPRESSION OF CERTAIN PARAMETERS OF MUST AND WINE COMPOSITION
Units
Metric system units of length (m), volume (l), and weight (g) are exclusively used. The conversion of metric units into Imperial units (inches, feet, gallons, pounds, etc.) can be found in the following enological work: Principles and Practices of Winemaking , R.B. Boulton, V.L. Singleton, L.F. Bisson, and R.E. Kunkee, 1995, The Chapman & Hall Enology Library, New York.
Expression of Total Acidity and Volatile Acidity
Although EU regulations recommend the expression of total acidity in the equivalent weight of tartaric acid, the French custom is to give this expression in the equivalent weight of sulfuric acid. The more correct expression in milliequivalents per liter has not come into widespread use. In this work, we have generally expressed acid levels as sulfuric acid, but in certain cases, the corresponding weight in tartaric acid, often used in other countries, has been given.
Using the weight of the milliequivalent of various acids, the table below can be used to convert from one expression to another.
| Coefficients of conversion from one expression of total or volatile acidity to another |
|
Desired expression |
| Known expression |
mEq/l |
g/l H 2SO 4 |
g/l tartaric acid |
g/l acetic acid |
| mEq/l |
1.00 |
0.049 |
0.075 |
0.060 |
| g/l H 2SO 4 |
20.40 |
1.00 |
1.53 |
1.22 |
| g/l tartaric acid |
13.33 |
0.65 |
1.00 |
|
| g/l acetic acid |
16.67 |
0.82 |
|
1.00 |
More particularly, to convert from total acidity expressed in H 2SO 4to its expression in tartaric acid, add half of the value to the original value:
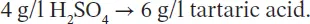
In the other direction, a third of the value must be subtracted.
The French also continue to express volatile acidity in equivalent weight of sulfuric acid. More generally, in other countries, volatile acidity is expressed as acetic acid. It is rarely expressed in milliequivalents per liter. The table above also allows simple conversion from one expression to another.
The expression of volatile acidity as acetic acid is approximately 20% higher than as sulfuric acid.
Evaluating the Sugar Concentration of Musts
This measurement is important for tracking grape ripening, monitoring fermentation kinetics, and, if necessary, determining the need for chaptalization.
This measurement is always determined by physical, densimetric, or refractometric analysis. The expression of the results can be given according to several scales: some are rarely used today (Baumé scale and Oechsle scale). At present, two systems exist (Volume 1, Section 10.4.3):
1 The potential ethanol content (PEC) of musts can be read directly on equipment that is graduated using a scale corresponding to 17.5 or 17 g/l of sugar for 1% volume of alcohol. Today, the EU recommends using 16.83 g/l as the conversion factor. The mustimeter is a hydrometer containing two graduated scales: one shows specific gravity and the other gives a direct reading of the PEC. Different methods varying in precision exist to calculate the PEC from a specific gravity reading. These methods take various elements of must composition into account (Boulton et al., 1995).
2 Degrees Brix express the percentage of sugar by weight. By multiplying degrees Brix by 10, the weight of sugar in 1 kg (or slightly less than 1 l) of must is obtained. A conversion table between degrees Brix and PEC is shown in Volume 1, Section 10.4.3. Seventeen (17) degrees Brix corresponds to an approximate PEC of 10%, and 20° Brix corresponds to a PEC of about 12%. Within the alcohol range most relevant to enology, degree Brix can be multiplied by 10 and then divided by 17 to obtain a fairly good approximation of the PEC.
In any case, the determination of the Brix or PEC of a must is approximate. First of all, it is not always possible to obtain a representative grape or must sample for analysis. Secondly, although physical, densimetric, or refractometric measurements are extremely precise and rigorously express the sugar concentration of a sugar and water mixture, these measurements are affected by other substances released into the sample from the grape and other sources. Furthermore, the concentrations of these substances are different for every grape or must sample. Finally, the conversion rate of sugar into alcohol (approximately 17–18 g/l per 1% alcohol by vol.) varies and depends on fermentation conditions and yeast properties. The widespread use of selected yeast strains has lowered the sugar conversion rate.
Measurements Using Visible and Ultraviolet Spectrometry
The measurement of optical density (or absorbance) is widely used to determine wine color (Volume 2, Section 6.4.5) and total phenolic compounds (Volume 2, Section 6.4.1). In these works, optical density is noted as OD: OD 420 (yellow), OD 520 (red), OD 620 (blue), or OD 280 (absorption in ultraviolet spectrum) to indicate the optical density at the indicated wavelengths.
Читать дальше