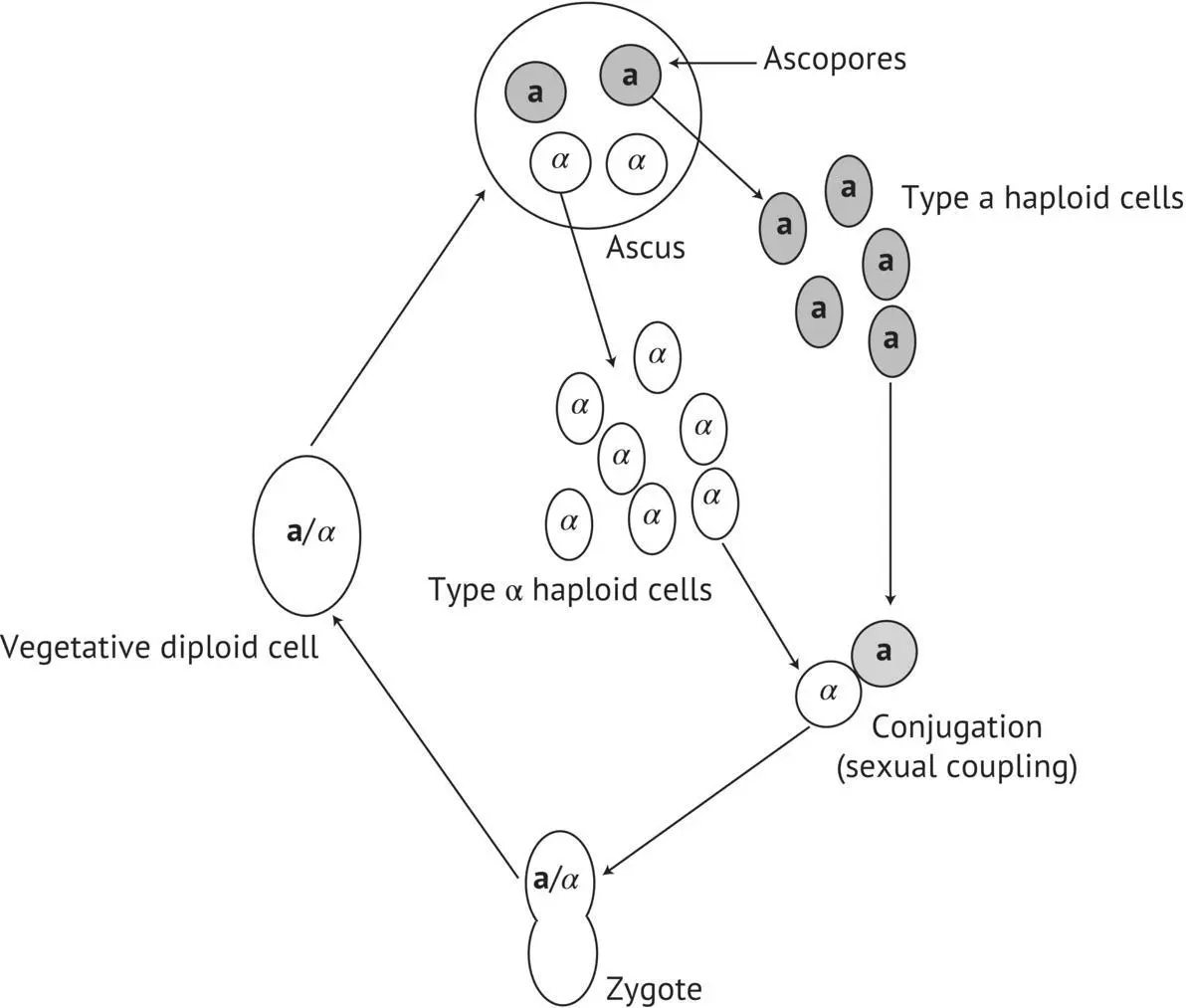
FIGURE 1.14 Reproduction cycle of a heterothallic yeast strain. a, α : spore mating types.
Most wild and selected winemaking strains that belong to the S. cerevisiae species are diploid and homothallic. This is also true of almost all of the strains that have been isolated in vineyards of the Bordeaux region. Moreover, recent studies carried out by Mortimer et al . (1994) in Californian and Italian vineyards have shown that the majority of strains (80%) are homozygous for the HO character ( HO / HO ); heterozygosis ( HO / ho ) is in the minority. Heterothallic strains ( ho / ho ) are rare (less than 10%). We have made the same observations for yeast strains isolated in the Bordeaux region; for example, the F10 strain, which is fairly prevalent in spontaneous fermentations in certain Bordeaux wines, is HO / HO . In other words, the four spores arising from an ascus give monoparental diploids, capable of forming asci when placed in a pure culture. This generalized homozygosis for the HO character of wild winemaking strains is probably an important factor in their evolution, according to the genome renewal phenomenon proposed by Mortimer et al . (1994) ( Figure 1.16). According to this author, the continuous reproduction of a yeast strain in its natural environment is accompanied by the accumulation of heterozygotic damage to the DNA. Certain slow‐growth or functional loss mutations of certain genes decrease strain vigor in the heterozygous state. Sporulation, however, produces haploid cells containing various combinations of these heterozygotic characters. All of these spores will become homozygous diploid cells with a series of genotypes because of the homozygosity of the HO character. Certain diploids that prove to be more vigorous than others will in time supplant the parents and less vigorous collaterals. This very persuasive model is supported by the characteristics of the wild winemaking strains analyzed. In these, the spore viability rate is an inverse function of the heterozygosis rate for a certain number of mutations. The completely homozygous strains present the highest spore viability and vigor.
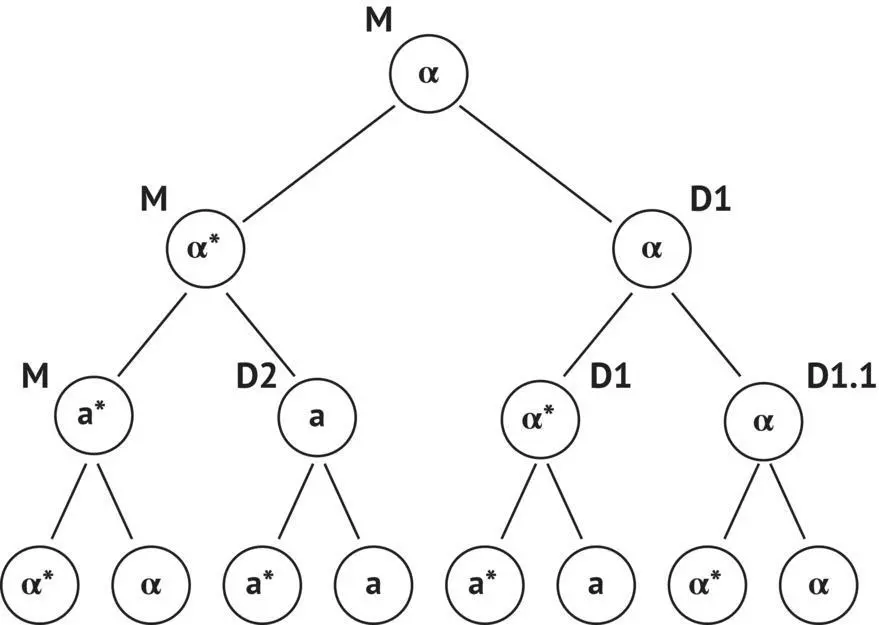
FIGURE 1.15 Mating type interconversion model of haploid yeast cells in a homothallic strain (Herskowitz et al ., 1992). * designates cells capable of changing mating type at the next cell division or cells already having undergone budding. M, initial cell carrying the HO gene; D1, D2, daughter cells of M; D1.1, daughter cell of D1.
In conclusion, we can question whether sporulation of strains under natural conditions is indispensable to ensure their growth and fermentation performance. We can also raise the question of conserving selected strains of active dry yeasts (ADYs) for use as yeast starters. It may be necessary to regenerate them periodically to eliminate possible mutations from their genome, which could diminish their vigor.
1.7 The Killer Phenomenon
1.7.1 Introduction
Certain yeast strains, known as killer strains (K), secrete protein toxins into their environment that are capable of killing other, sensitive strains (S). The killer strains are not sensitive to their own toxin but can be killed by a toxin that they do not produce. Additionally, neutral strains (N) do not produce a toxin but are resistant. The action of a killer strain on a sensitive strain is easy to demonstrate in the laboratory on an agar culture medium at pH 4.2–4.7 at 20°C. The sensitive strain is inoculated into the mass of agar before it solidifies; then the strain to be tested is inoculated in streaks on the solidified medium. If it is a killer strain, a clear zone in which the sensitive strain cannot grow encircles the inoculum streaks ( Figure 1.17).
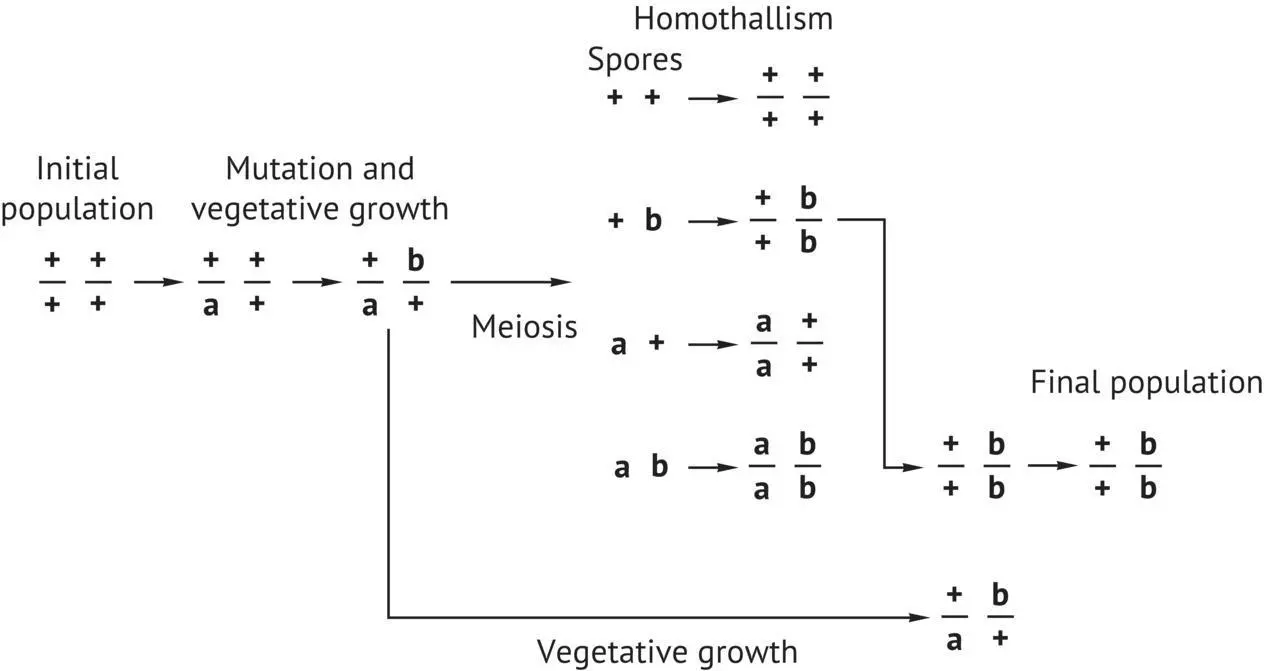
FIGURE 1.16 Genome renewal of a homozygote yeast strain for the HO gene of homothallism, having accumulated recessive mutations during vegetative reproduction (Mortimer et al ., 1994). (a and b), mutation of certain genes.
This phenomenon, known as the killer factor, was discovered in S. cerevisiae , but killer strains also exist in other yeast genera such as Hansenula , Candida , Kloeckera , Hanseniaspora , Pichia , Torulopsis , Kluyveromyces , and Debaryomyces . Killer yeasts have been classified into 11 groups according to the sensitivity reaction between strains as well as the nature and properties of the toxins involved. The killer factor is a cell interaction model mediated by the excreted protein toxin. It has given rise to much fundamental research (Tipper and Bostian, 1984; Young, 1987). Barre (1984, 1992), Radler (1988), and Van Vuuren and Jacobs (1992) have described the technological implications of this phenomenon for wine yeasts and the fermentation process.
1.7.2 Physiology and Genetics of the Killer Phenomenon
The determinants of the killer factor are both cytoplasmic and nuclear. In S. cerevisiae , the killer phenomenon is associated with the presence of double‐stranded RNA particles, called virus‐like particles (VLP), in the cytoplasm. They are in the same category as noninfectious mycoviruses. There are two kinds of VLP: M and L. The M genome (1.3–1.9 kb) codes for the toxin (K) and for the immunity factor (R). The L genome (4.5 kb) codes for an RNA polymerase and the protein capsid that encapsulates the two genomes. Killer strains (K +R +) secrete the toxin and are immune to it. The sensitive cells (K −R −) do not possess M VLP, but most of them have L VLP. The two types of viral particles are necessary for the yeast cell to express the killer phenotype (K +R +), since the L mycovirus is necessary for the maintenance of the M type.
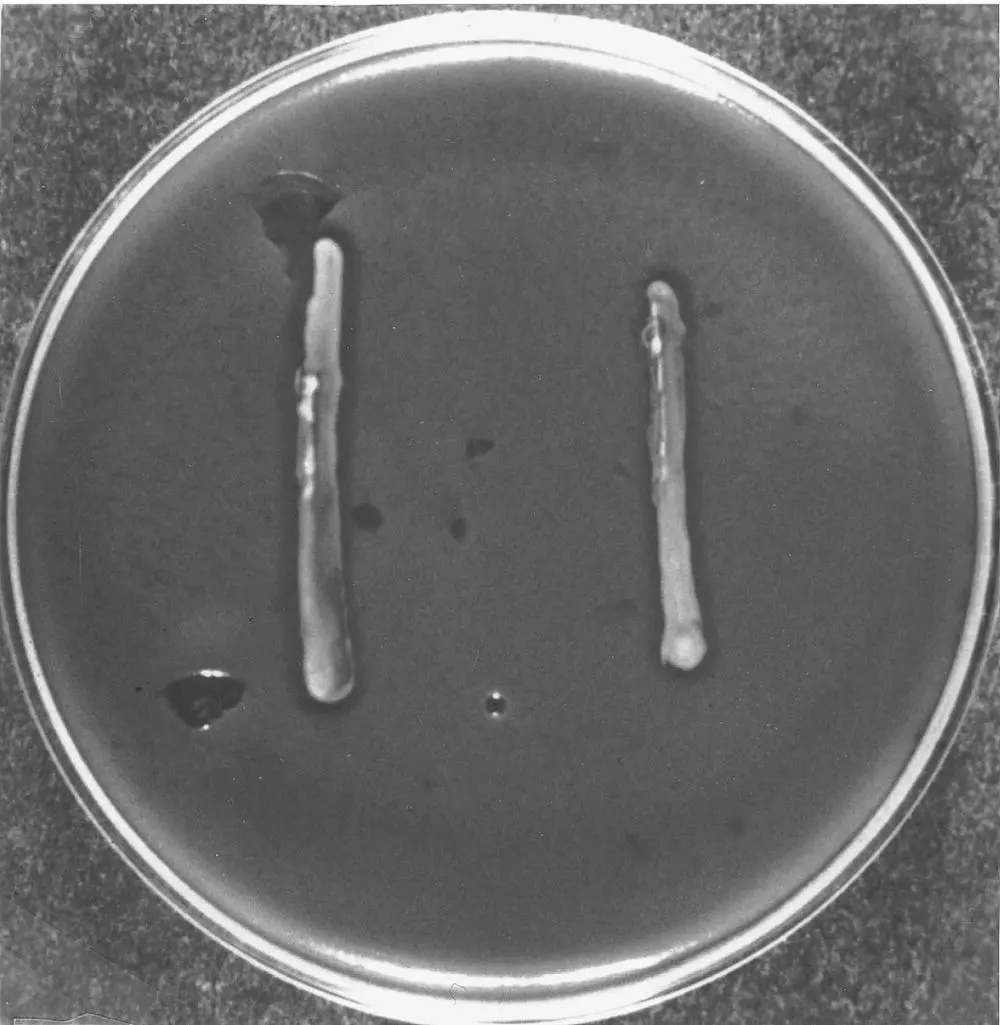
FIGURE 1.17 Identification of the K2 killer phenotype in S. cerevisiae . The presence of a halo around the two streaks of the killer strain is due to the death of the sensitive strain cultivated in the medium.
There are four types of killer activity in S. cerevisiae strains, corresponding to K1, K2, K28, and Klus toxins. They are, respectively, coded by M1, M2, M28, and Mlus VLPs with sizes of 1.8, 1.7, 2.1, and 2.3 kb. The K2 activity group is by far the most widespread in the S. cerevisiae strains encountered in wine (Rodríguez‐Cousiño et al ., 2011). Neutral strains (K −R +) are insensitive to a given toxin without being capable of producing it. They possess M VLPs of normal size that code only for the immunity factor, but they either do not produce toxins or are inactive because of mutations affecting the M‐type RNA.
Many chromosomic genes are involved in the maintenance and replication of L and M RNA particles as well as in the maturation and transport of the toxin produced.
The K1 toxin is a small protein made up of two subunits (9 and 9.5 kDa); it is active and stable in a very narrow pH range (4.2–4.6). It is therefore inactive in grape must. The K2 toxin, a 16 kDa glycoprotein produced by homothallic strains of S. cerevisiae encountered in wine, is active between pH 2.8 and 4.8 with a maximum activity between 4.2 and 4.4. It is therefore active at the pH of grape must and wine.
Читать дальше
















