Gross findings: The gross lesions of CIRD typically reflect the aerogenous introduction of bacteria into the pulmonary tissues. The lungs are congested and consolidated, most consistently within the cranial and ventral portions of the cranial lobes. There can be pleural mottling that may involve multiple lobes. Depending upon whether a viral component is acute or chronic, the bronchopneumonia could be superimposed on a more diffuse pattern of lung involvement (interstitial pneumonia). The thoracic cavity should be examined for excessive pericardial and thoracic fluid production (normal is less than 5 ml in both cavities), +/− concurrent pericarditis and pleuritis.
Histological, microbiological (lung), and molecular diagnostic (lung) samples should be taken according to the general respiratory disease protocol above.
5.5.3.2.3 Canine Influenza: (CIV)
As with most viral diseases, it can be difficult to determine the role of a virus in a clinical event. Reliable diagnosis of many viral diseases by serology requires both acute and convalescent serum samples. Virus detection by PCR in respiratory secretions from acutely ill or recently exposed animals is possible, but false negatives are not uncommon. The Becton‐Dickinson Flu‐A ELISA test may also be used on nasal secretions from acutely affected animals. While the mortality rate of canine influenza is fairly low (5–8%), if an animal does die or is euthanized with respiratory disease, the most accurate test for the virus is a PCR test of respiratory tissue. This, combined with histological features of viral‐induced pneumonia, would be the “gold‐standard” for the presence, and effect, of the virus. In several identified cases of CIV, there was a concurrent and severe bacterial pneumonia, so samples for culture and antibiotic sensitivity should be concurrently submitted.
Gross findings: Influenza virus will be hematogenously disseminated (affect all lobes). The lungs can be hemorrhagic or consolidated (interstitial pneumonia). Again, lungs are among the most difficult of organs in which to detect gross changes; histological analysis is of paramount importance in evaluating the sequelae of the virus and/or co‐infections in the lungs. The tissue collection protocol that was elaborated for respiratory disease sample collection should be followed.
PCR: Fresh, or fresh frozen respiratory tissue is always best, but RNA extracted from paraffin‐embedded tissues has been used to detect the virus. Laboratories that offer this type of testing are limited but will accept samples by courier. Some specific instructions are listed below.
US:
https://www.idexx.com/en/veterinary/reference‐laboratories/canine‐feline‐influenza
California:
https://pcrlab.vetmed.ucdavis.edu/veterinary‐diagnostics
New York:
https://ahdc‐portal.vet.cornell.edu/#!/test_fee/search
Wisconsin:
https://www.wvdl.wisc.edu/index.php/canine‐influenza‐information‐for‐veterinarians
5.5.3.2.4 Feline infectious respiratory disease (FIRD) (Upper respiratory infection (URI))
FIRD is typically multifactorial, and, like CIRD, recognition of the contributory infectious agents can be very helpful in organizing a response. Moreover, unusual agents, or unusually virulent agents have plagued some shelters. The most commonly recognized agents involved in infections limited to the upper respiratory tract are feline calicivirus (FCV), feline herpesvirus (FHV1), Mycoplasma , Chlamydia , and occasionally B. bronchiseptica ( Pesavento and Murphy 2014 ) . Other bacterial organisms such as Streptococcus canis have also and more recently been described in outbreak situations (Pesavento et al. 2007). Nasal swabs can be performed postmortem, and sampling in an outbreak of severe FIRD should include the histological samples described in the general section on respiratory disease. Many diagnostic laboratories now offer PCR panels for the most common organisms associated with FIRD. Nonetheless, the presence of the pathogen does not always imply causation, and concurrent culture and histology can be very helpful in identifying the cause. Gross assessment cannot distinguish among even the most common agents involved in FIRD, however, it is helpful to note the character of the nasal conchae, lungs, and whether or what type of fluid is present within the sinuses.
5.6 Other Shelter Necropsies
5.6.1 Necropsy on a Previously Healthy Animal Found Dead
5.6.1.1 Acute Death
Common causes of acute death, with special attention to possible shelter situations or submissions are anaphylaxis, physical trauma (with neurologic or hemorrhagic consequences), intestinal malpositions (volvulus, intussusception), cardiomyopathy, electrocution (lightning or chewing on electric cords), gunshot, drowning, septicemia, heatstroke, dehydration, or ingestion of toxins or poisons (plant or synthetic, including disinfectants). Note that the most common cause of acute death in incompletely vaccinated shelter cats is panleukopenia. While some of these conditions may be obvious on gross necropsy (physical trauma, intestinal malpositions, gunshot, cardiomyopathy), in others histopathology is useful (e.g. some toxins, septicemia); still others are unlikely to have either gross or histological lesions (e.g. anaphylaxis, dehydration, electrocution, some toxins, heat stroke). Even in this latter subset, a gross necropsy effectively rules out most possible causes of disease. In all cases, diagnosis requires a good history to arrive at the definitive diagnosis.
5.6.2 Feline Infectious Peritonitis (FIP)
There is no single, predictable target organ for feline infectious peritonitis (FIP). The virus widely (systemically) disseminates in macrophages, and the clinical outcome is dependent on both the host immunity and the specific organ affected. Histopathology on biopsy or necropsy specimens remains the gold standard for diagnosis. Many of the pre‐mortem tests, and especially a cumulative amount of information, can be highly suggestive of the disease. If an animal dies or is euthanized with suspect FIP, a necropsy with histology would be diagnostic.
Gross findings: In the effusive form, there will be fluid within either or both the thoracic and abdominal cavities. The fluid is high in protein and may vary from slightly viscous to gelatinous. The surfaces of the viscera are covered with tiny (1–5 mm) friable, pale tan to white plaques (fibrin) that can give the surfaces a granular appearance (See Figure 5.9).
In the non‐effusive (chronic) form, there will be nodules (granulomas) of variable size present in one or multiple organs. These can vary in color from off white to light tan, in texture from slightly firm to soft. The nodules are typically associated with capsular or serosal vessels, although when abundant this can be difficult to distinguish. Within organs, the granulomas can be scattered throughout the parenchyma. Lymph nodes are often enlarged.
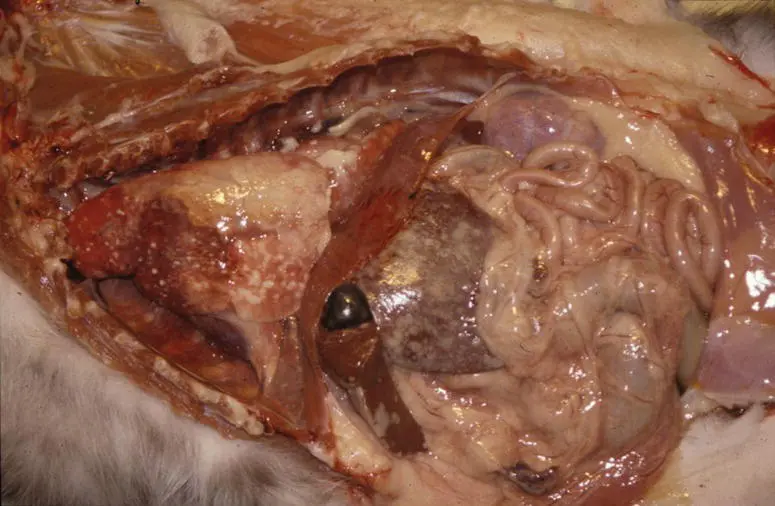
Figure 5.9Feline infectious peritonitis (FIP) virus (feline coronavirus). In the effusive form of FIP, along with intracavitary fluid, the surfaces of abdominal and thoracic viscera are covered with small (1–2 mm) to coalescing pale tan plaques.
Formalin‐fixed tissues: Samples should be taken from affected organs (in the case of the non‐effusive form, this would be any viscera with detectable granulomas). In the case of the effusive form, multiple samples should be taken from affected viscera (liver, GI, lung). If the clinical presentation is limited to the nervous system and FIP is suspected, it is imperative to submit the brain. Brain lesions are, however, rarely uniquely present.
Читать дальше













