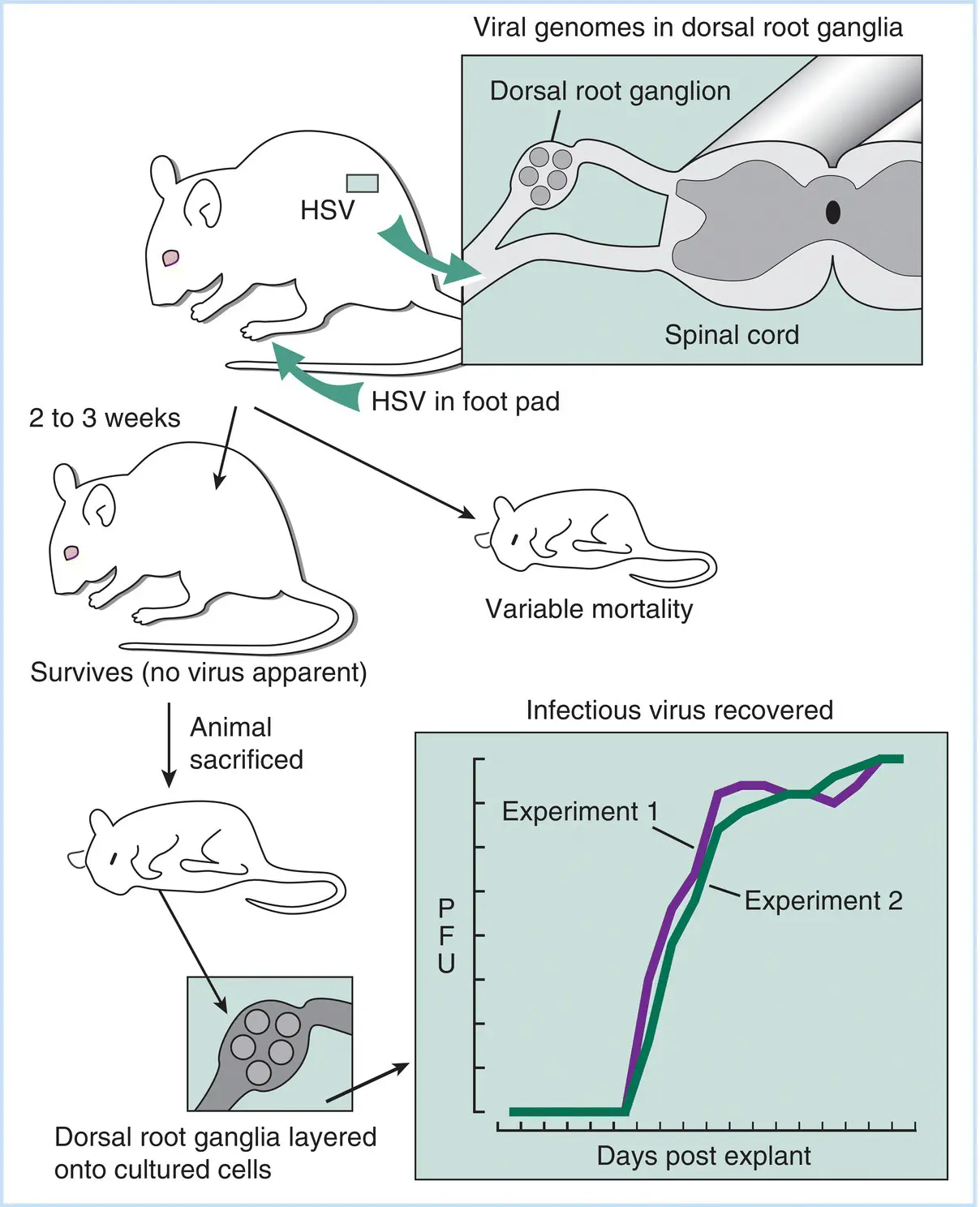
Figure 3.6Analysis of the establishment and maintenance of latent HSV infections in mice. A number of mice are inoculated in the footpad, and following the symptoms of primary disease, which include foot swelling and minor hindquarter paralysis, many mice recover. Those that do not recover have infectious virus in their CNS. The mice that recover are latently infected and no infectious virus can be detected, even with high‐sensitivity measurements of nervous and other tissue. HSV genomes, but not infectious virus, can be detected in nuclei of sensory nerve dorsal root ganglia. When these ganglia are cultured with other cells that serve both as an indicator of virus replication and as a feeder layer for the neurons (i.e., explanted), a significant number demonstrate evidence of virus infection and infectious virus can be recovered, as shown on the inset graph (two separate experiments are shown, with essentially the same results).
Guinea pigs are favored experimental animals for the study of infection and disease because they are readily infected with many human pathogens. They are an important model for the study of HSV‐2, which cannot be studied effectively in the murine and rabbit models just described.
Guinea pigs can be infected vaginally with inoculation of virus, and following a localized infection, latency can be established. As occurs in the murine and rabbit models, virus or viral DNA can be recovered from latently infected neurons (those enervating the vaginal area in this case). As in rabbits, latent infection in guinea pigs will spontaneously reactivate, and periodic examination can be used to measure reactivation rates. Unlike rabbits, however, guinea pig reactivation cannot be induced. Also, HSV‐2 reactivates much more frequently than does HSV‐1 in the guinea pig model; therefore, this model may be of some value in establishing the subtle genetic differences between these two types of viruses that manifest as a differential tropism for mucosa.
1 In the case of rabies virus, how would you classify humans with respect to their role as a host?
2 What characteristics are shared by all hepatitis viruses?
3 Using the data presented in Table 3.1, answer the following questions:Which of the viruses in the table are vectored by mosquitoes?Which of the viruses in the table are transmitted in an aerosol?Which of the viruses in the table are transmitted by injection of blood?Which of the viruses in the table are neurotropic?
4 You are a viral epidemiologist studying the population of Spitzbergen Island off the coast of Norway (see Figure 3.2). Suppose that a team of scientists plans to visit this island by special boat during the Christmas holiday season. How might this visit change the pattern of respiratory infections you have been observing? What criteria must exist for this visit to have an effect on the pattern of viral respiratory illness on the island?
5 You have isolated two mutant strains of virus Z – mutant 1 and mutant 2. Neither strain can replicate when infected into cells, but either can be propagated in cell culture when coinfected with mutant virus 3. When you coinfect cultured cells with mutants 1 and 2 together, infection proceeds, but only mutant 1 and mutant 2 can be recovered from the infected cells. What is the best explanation of these results?
CHAPTER 4 Patterns of Some Viral Diseases of Humans
THE DYNAMICS OF HUMAN–VIRUS INTERACTIONS
The stable association of viruses with their natural host places specific constraints on the nature of viral disease and mode of persistence
Classification of human disease–causing viruses according to virus–host dynamics Viral diseases leading to persistence of the virus in the host are generally associated with viruses having long associations with human populations Viral diseases associated with acute, severe infection are suggestive of zoonoses
PATTERNS OF SPECIFIC VIRAL DISEASES OF HUMANS
Acute infections followed by virus clearingColds and respiratory infections Influenza Variola
Infection of an “accidental” target tissue leading to permanent damage despite efficient clearing
Persistent viral infections Papilloma and polyomavirus infections Herpesvirus infections and latency Other complications arising from persistent infections
Viral and subviral diseases with long incubation periods Rabies HIV/AIDS Prion diseases
SOME VIRAL INFECTIONS TARGETING SPECIFIC ORGAN SYSTEMS
Viral infections of nerve tissue
Examples of viral encephalitis with grave prognosisRabies Herpes encephalitis
Viral encephalitis with favorable prognosis for recovery
Viral infections of the liver (viral hepatitis) Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E
QUESTIONS FOR CHAPTER 4
THE DYNAMICS OF HUMAN–VIRUS INTERACTIONS
We have seen that the process of infection and consequent disease is controlled by a number of factors ranging from the effect of specific genes controlling aspects of pathogenesis to more subjective factors that can be classified as important in overall virulence of the disease. The nature of the viral disease – or more accurately, the viral infection, its severity, the fate of the host, and the fate of the virus causing the disease – is important from a purely medical point of view. But, as importantly, the features of the dynamic interaction between virus and host provide important clues as to how long a particular virus has been associated with its host. Further, the nature of the interaction provides clues as to the evolutionary history of the host.
The stable association of viruses with their natural host places specific constraints on the nature of viral disease and mode of persistence
As noted in Chapter 3, viruses are maintained by active rounds of infection somewhere in their reservoir. We have seen that a virus infection leading to immunity to re‐infection will lead to virus extinction in a small population once the pool of susceptible individuals is exhausted. Also, even in a large reservoir, if the virus infection directly or indirectly leads to death of a large enough number of individuals, the host population of the reservoir will crash and, in extreme cases, may become extinct. Clearly, a virus that can only replicate within that population will also become extinct. These limitations, which can be described with precision using the mathematics of population biology and epidemiology, lead to a number of evolutionary constraints on the dynamics of the virus–host interaction. Viruses whose infection leads to an acute disease followed by clearing and immunity need a large host population, while the outcome of the disease cannot be too lethal or the virus cannot be maintained. If infection results in mild or inapparent symptoms, however, there still must be efficient spread. This latter pattern of virus infection is a common feature of viruses with animal reservoirs that contain large populations, such as flocks of migratory birds or herds of ungulates. Since the agricultural/urban revolution starting about 10 000 years ago, which engendered rapid increases in our population, urbanized human populations fit these criteria also.
The early history of humans, however, was not one of large sedentary populations. Rather, our ancestors lived in small, nomadic groups organized along familial lines. This organization is similar in broad outline to that of predators such as wolves and large cats. In such a population, no virus that is cleared with resulting immunity can persist; therefore, only a virus that can establish a persistent infection of its host, with little or no diminution of the host's ability to survive and propagate, can persist. Furthermore, this persistence must allow for infectious virus to be present at opportune moments for infection of new, susceptible individuals (i.e., infants and occasional adults encountered from other groups). A number of viruses have replication strategies and genetic capacities to establish such infections, and it is striking that genetic analysis of such viruses demonstrates ancient associations with humans.
Читать дальше













