Rabies: where is the virus during its long incubation period?
Rabies and its transmission by the bite of infected animals to other animals and humans are well known in almost all human cultures. The disease and its transmission were carefully described in Arabic medical books dating to the Middle Ages, and there is evidence of the disease in classical times. One of the puzzles of rabies virus infection is the very long incubation period of the disease. This long period plays an important role in the mechanism of spread, and it is clear that animals (or humans) infected with the virus can be vaccinated weeks to months after infection and still mount an effective immune response.
The pathogenesis of rabies has been studied for over a century, and our current understanding is well founded in numerous careful studies made at varying levels of sensitivity using a number of approaches. An example of the use of immunological methods is shown in Figure 3.5. The basic course of infection starts with inoculation of virus at a wound caused by an infected animal, followed by limited virus replication at the site of primary infection. For the disease to develop, the virus must enter a neuron at a sensory nerve ending. These sensory nerve endings exist in all sites where the virus is known to enter an animal. Following this, the virus spreads passively to the nerve cell body in a dorsal root ganglion, where it replicates to a high level. Either this replicated virus, or other virus moving directly, passes into neurons of the cerebellum and cerebral cortex, where it replicates to high levels. Such replication leads to distinct behavioral changes associated with virus transmission. The virus also moves away from the central nervous system (CNS) to sensory neurons and salivary glands of the oral mucosa, where it replicates and is available for injection into another animal.
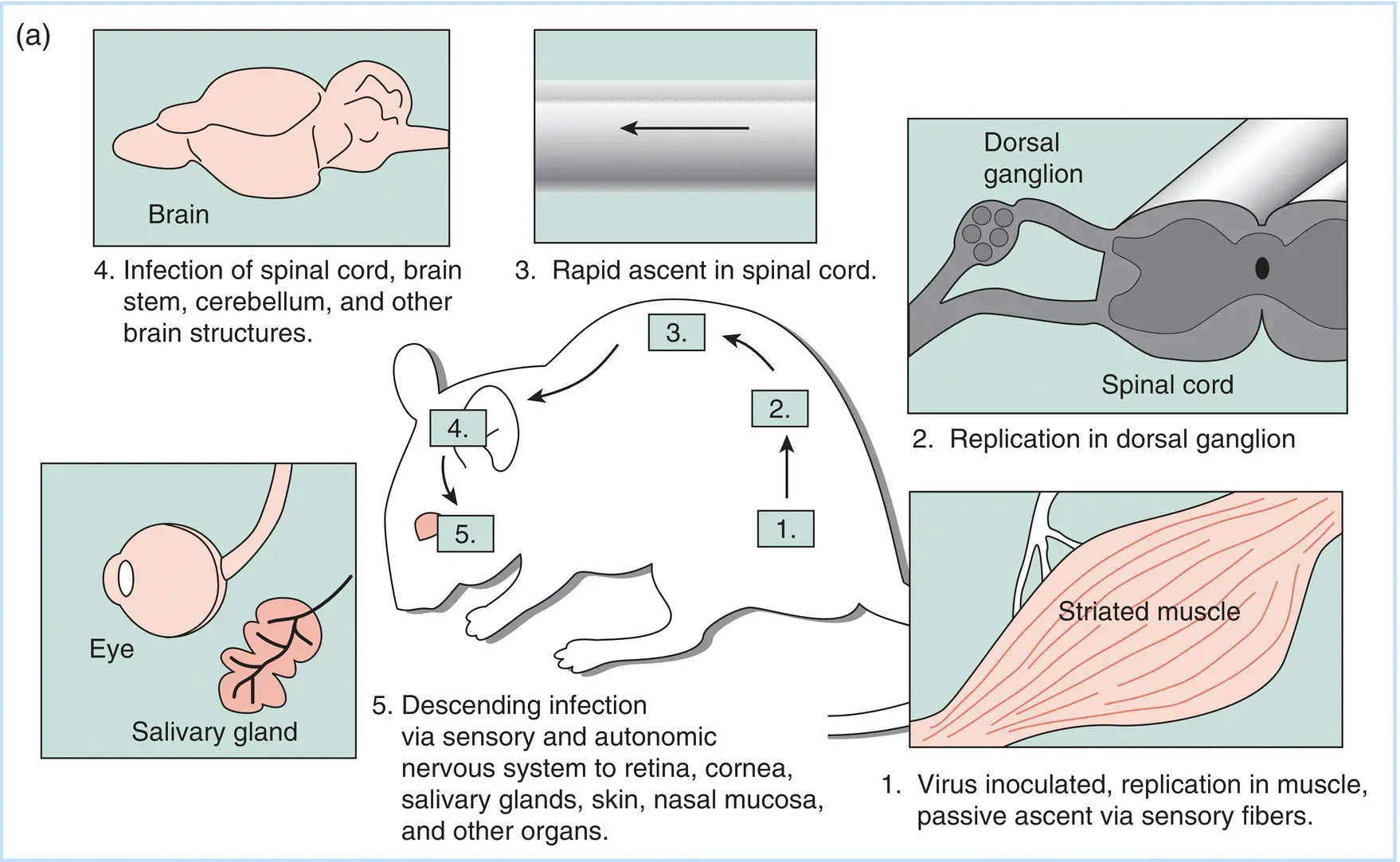
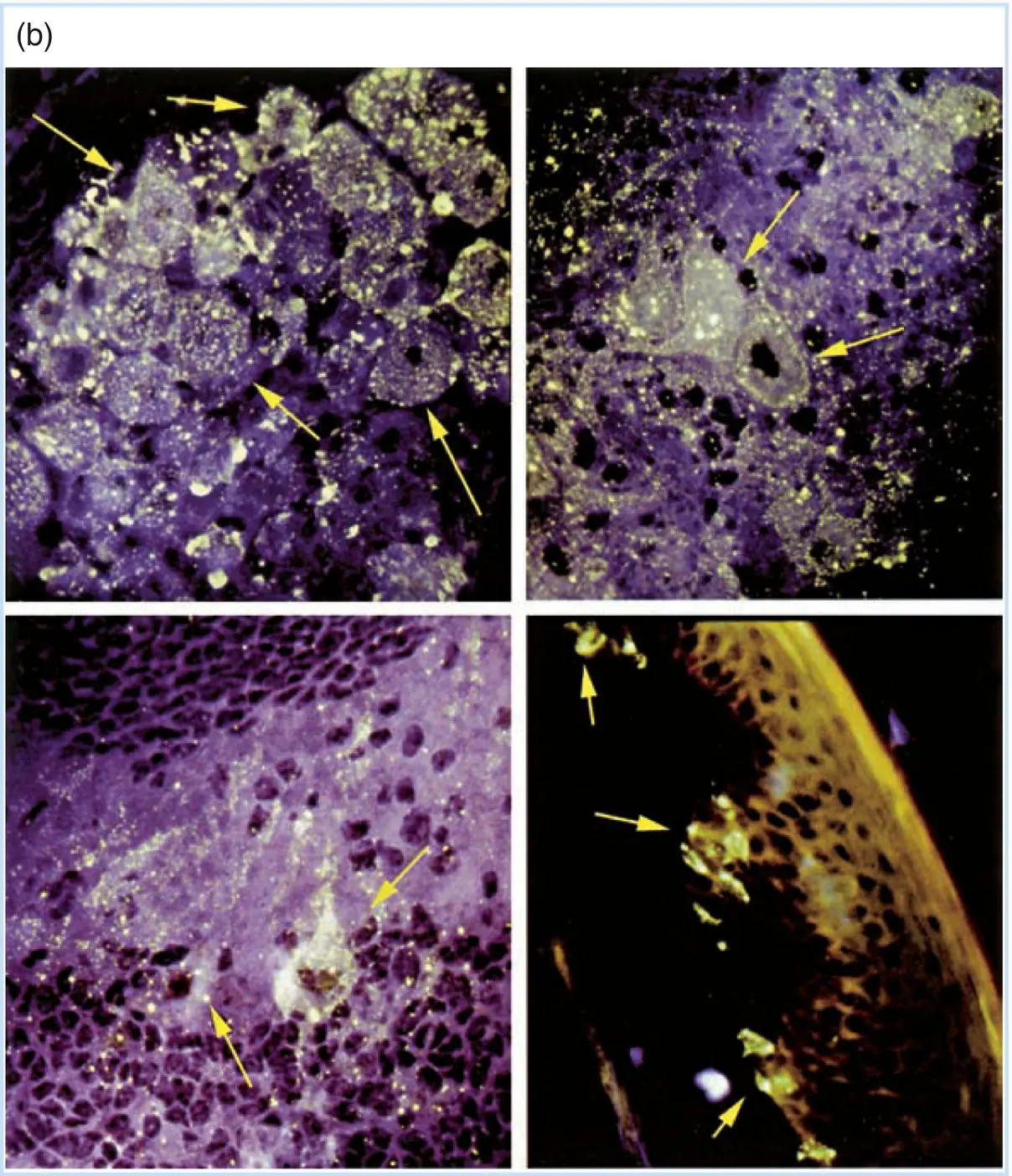
Figure 3.5Visualization of rabies virus–infected neurons in experimentally infected animals. (a) A schematic representation of the pathogenesis of rabies in an experimentally infected laboratory animal. (b) Immunofluorescent detection of rabies virus proteins in neurons of infected animals. As described in Chapters 7and 12, the ability of an antibody molecule to specifically combine with an antigenic protein can be visualized in the cell using the technique of immunofluorescence. The cell and the antibody bound to it are then visualized in the microscope under ultraviolet light, which causes the dye to fluoresce (a yellow‐green color). The top left panel shows replication of rabies virus in a sensory nerve body in a dorsal root ganglion along the spine of an animal infected in the footpad. The bottom left panel shows the virus replicating in a neuron of the cerebellum, while the top right panel shows infected neurons in the cerebral medulla. Infection of the brain leads to the behavior changes so characteristic of rabies infections. Finally, the sensory nerve endings in the soft palate of a hamster infected with rabies virus at a peripheral site contain virus, as shown by the fluorescence in the bottom right panel. This virus can move to the saliva, where it can be spread to another animal. The arrows point to selected cells showing the variation in signal intensity that is typical of infections in tissues.
As early as 1887, CNS involvement was shown to result from direct spread of the virus from the site of infection into the CNS, as experimental animals that had their sciatic nerve severed prior to injection of the footpad with rabies virus did not develop the disease. The following experiment showed that the virus can remain localized at the site of infection for long periods of time: The footpad of several experimental animals was injected with virus at day 0 and then the inoculated foot was surgically removed from different groups at days 1, 2, 3, and so on after infection. Mice whose foot was removed as long as three weeks after infection survived without rabies, but once neurological symptoms appeared, the mice invariably died. Since removal of the foot saved the mice, it is clear that the virus remained localized there until it invaded the nervous system.
Finally, a similar experiment showed that rabies virus virulence for a specific host could be increased by multiple virus passages(rounds of virus replication) in that host. Virus isolated from a rabid wild animal takes as long as a week to 10 days to spread to the CNS of an experimentally infected laboratory animal. In contrast, isolation of virus from animals developing disease and re‐inoculation into the footpad of new animals several times result in a virus stock that can invade the test animal's CNS in as little as 12–24 hours. Further, the virus stock that has been adapted to the laboratory animal is no longer able to efficiently cause disease in the original host. As described in Chapter 8, this is one way of isolating strains of virus that are avirulent for their natural host and have potential value as vaccines.
Herpes simplex virus latency
There are two closely related types of herpes simplex virus: type 1 (facial, HSV‐1) and type 2 (genital, HSV‐2). Both establish latent infections in humans, and reactivation from such infections is important to virus spread. Some details concerning latent infection by HSV are discussed in Chapter 18in Part IV. Different animal models demonstrate both general similarities and specific differences. These differences illuminate a major limitation of many animal models for human disease: A model often only partially reflects the actual course of disease in the natural host – in this case, in humans.
HSV infection in the eye or the footpad of mice can lead to a localized infection with spread of virus to the CNS and then to the brain. Although some animals die, as shown in Figure 3.6, survivors maintain a latent infection in sensory nerve ganglia. During this latent infection, no infectious virus can be recovered from nerve tissue, but if the nerve ganglia are explanted(dissected, dissociated, and maintained on a feeder layerof cultured cells), virus will eventually appear and begin to replicate. This observation demonstrates both that the viral genome is intact in the latently infected neuron, and that virus is not present in infectious form until something else occurs.
This model is quite useful for the study of genetic and other parameters during establishment and maintenance of a latent infection. For example, the sensory neurons can be isolated and viral DNA can be recovered. But since mice do not efficiently reactivate HSV, the physiological process of reactivation, where virus can be recovered at the site of initial infection, cannot be effectively studied in mice.
Infection of rabbit eyes with HSV leads to localized infection and recovery. The rabbits maintain virus in their trigeminal ganglia, and viral DNA, virus, or both can be recovered using methods described for the murine model. Unlike mice, rabbits spontaneously reactivate HSV, and virus occasionally can be recovered from the rabbit's tear film. Further, this reactivation can be induced by iontophoresisof epinephrine with high frequency. Rabbits, because HSV can reactivate in them, are vital to the design of experiments to investigate induced reactivation, although they are more expensive to purchase and keep than mice.
Читать дальше














