While many human viral diseases are maintained in the human population itself, some important pathogens are maintained primarily in other vertebrates. A disease that is transmissible from other vertebrates to humans is termed a zoonosis . Rabies is a classic example of a zoonosis that affects humans only sporadically. Because humans rarely transmit the virus to other animals or other humans, infection of a human is essentially a dead end for the virus. The rabies virus, which is transmitted in saliva via a bite, is maintained in populations of wild animals, most generally carnivores. The long incubation period and other characteristics of the pathogenesis of rabies mean that an infected animal can move great distances and carry out many normal behavioral patterns prior to the onset of disease symptoms. These symptoms may include hypersensitivity to sound and light, and finally, hyperexcitability and frenzy. Except in rare instances of inhalation of aerosols, humans only acquire the disease upon being bitten by a rabid animal; however, the fact that the disease can be carried in domestic dogs and cats means that when unvaccinated pets interact with wild animal sources, the pets become potential vectors for transmission of the disease to humans. Vaccination of pets provides a generally reliable barrier.
Many viral zoonoses require the mediation of an arthropod vector for spread to humans. The role of the arthropod in the spread can be mechanical and passive in that it inoculates virus from a previous host into the current one without virus replication having occurred (a favored route with animal poxviruses), but the arthropod's role as a vector can be dynamic. For viruses with RNA genomes that are transmitted between hosts via arthropods (such as those responsible for yellow fever, a number of kinds of encephalitis, dengue fever, and Zika virus), virus replication in the vector provides a secondary reservoir and a means of virus amplification. This makes spread to a human host highly efficient, since even a small inoculation of the virus into the arthropod vector can result in a large increase in virus for transmission to the next host.
Most (but certainly not all) virus infections induce an effective and lasting immune response. Some of the basic features of this response are described in Part II, Chapters 7and 8. An effective immune response means that local outbreaks of infection result in the formation of a population of resistant hosts – often termed herd immunity. This means that any virus that induces protective immunity must maintain itself either in another reservoir or by dynamically spreading in “waves” through the population at large. If enough members of the susceptible population become immune, virus cannot spread effectively and it becomes extinct. This herd immunity is a major factor in both gradual and abrupt acquisition of genetic alterations that create new serotypes of viruses that can escape immunity to the original strain.
Viral epidemiology in small and large populations
The occurrence of mild respiratory infections (such as a common cold) in isolated communities provides graphic examples of the process of virus extinction. For example, when scientists visit the Antarctic research stations at the beginning of the Antarctic summer, they bring in colds to infect the resident population. When scientists stop arriving with the onset of winter, the prevailing respiratory diseases run their course and disappear. Figure 3.2charts a classic epidemiological study of respiratory illness in an isolated fishing and mining population on Spitzbergen Island, Norway, in the Arctic Ocean. Note that after the last contact with the “outside world,” the incidence of such viral‐borne respiratory infections rapidly declines to an undetectable level.
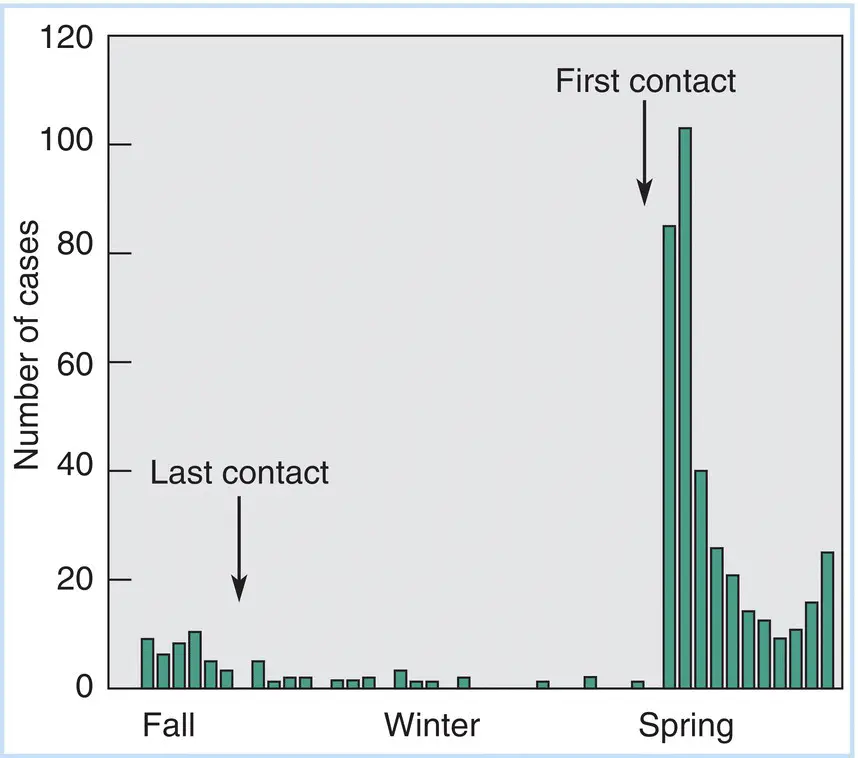
Figure 3.2Occurrence of respiratory illness in an arctic community (Spitzbergen Island, Norway) that is isolated during the winter months. Following the last boat communication with the European mainland, the number of respiratory illnesses declines from a low number to almost nil. With the first boat arriving in the spring, new serotypes of respiratory viruses are communicated from the crew and passengers, and a “mini‐epidemic” occurs. As the virus passes through the population, resistance builds and infections decline to a low level.
Source: Based on data originally published by Paul, J.H. and Freese, H.L. (1933). An epidemiological and bacteriological study of the “common cold” in an isolated Arctic community (Spitsbergen [sic]). American Journal of Hygiene 7:517.
In large populations, the rate of virus spread greatly surpasses the limitations of the generation of herd immunity, and the introduction of a novel pathogenic virus leads to epidemic spread of disease. The outbreak of severe acute respiratory syndrome (SARS) in China in the early part of the 2000s and its spread to Canada provide an important case study of this process, as well as providing examples of effective and ineffective public health measures set up to deal with it. The SARS virus is a member of the coronavirus family and distantly related to one that causes mild colds in humans. The virus appears to have been maintained in wild animal populations in southeast China and was introduced into humans in Guangdong Province and the city of Guangzhou (Canton) through the custom of using such animals as dietary delicacies. While human infection is characterized by flu‐like symptoms, the persistence, severity, and relatively high death rate suggested that this was a novel type of infection – a novel virulent form of influenza or an uncharacterized respiratory virus. Evidence suggests that the Chinese government, in hopes of avoiding loss of tourist and business travel revenues, suppressed news of this outbreak.
The disease was spread by a physician who had treated infected individuals in China and then traveled to Hong Kong on business – as the first identified source of infection, he was termed the index case. He contaminated the registration desk of the hotel in which he was registered, and this desk served as a source of infection for a number of tourists from other parts of the world (including Toronto, Canada) who happened to be staying in the same hotel. The disease spread to individuals in Hong Kong and was eventually described and quarantined there, but not before other infected individuals traveled back to Canada and, in lesser numbers, to the United States.
In Toronto, the index case of the local epidemic was a woman who infected her immediate family members upon returning from Hong Kong. She and one son subsequently died, but not before being admitted to the hospital where a physician treating them as well as other members of the hospital staff were infected. This illustrates a continuing conundrum of modern medicine – the concentration of individuals suffering from an infectious disease in a hospital can serve as a potent reservoir for the spread of that disease through the staff attending them and, subsequently, others. Such nosocomialinfections are a major occupational hazard for hospital personnel as well as patients suffering other maladies, yet hospitals are obviously necessary for the treatment of the severely ill.
The Canadian public health authorities were reluctant to initiate stringent quarantines for infected individuals in the hospital where the first patients were housed, and the hospital served as a source of infected individuals who spread the disease to others both through family and social contacts and through contact in other workplaces. In contrast, in the United States, the infection was initiated somewhat later. By that time sufficient information concerning the disease, its spread, and its control led to rapid quarantine of SARS patients, especially among health workers. These control methods were successful in the United States and Europe, as well as in Hong Kong, and the virus never spread beyond the first intimate contacts.
Читать дальше













