Teleost skulls are a complicated series of bones; anatomy varies significantly between species. Vertebrae also vary across species. Radiographically, vertebrae usually have a prominent cross that represents the conical recesses enclosing the intervertebral pad, a neural spine, a hemal arch (or pleural ribs cranially), and a hemal spine (Roberts and Ellis 2012). Ribs are either pleural (attached to vertebrae) or intermuscular (within muscular tissue) as in salmonids (Helfman et al. 2009).
Fin shapes and locations vary between species. They may be embedded in musculature or bone. Firm fin spines are common, particularly along the dorsal fin, and present a human health hazard. Some fin spines also contain venom, e.g. lionfish ( Pterois spp.) and stonefish ( Synanceiidae ). Some fins are modified into suckers, e.g. lumpfish ( Cyclopteridae ). The lobe‐finned fish, lungfish ( Dipnoi ) and coelacanths ( Latimeria spp.), have muscular fins with an articulating bone in their pectoral fin.
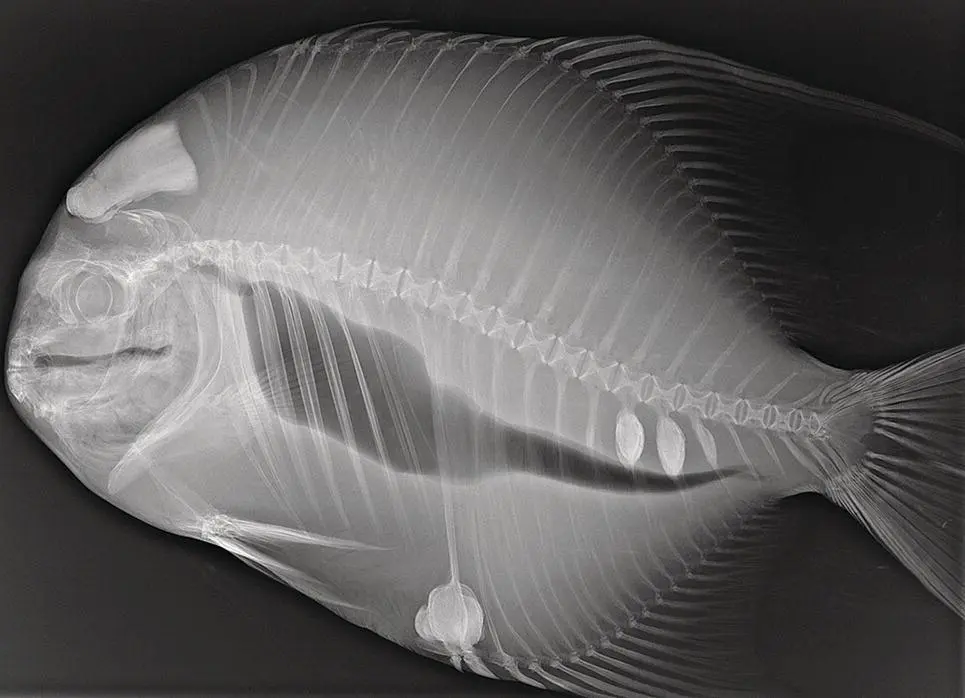
Figure A1.2 Radiograph of an Atlantic spadefish ( Chaetodipterus faber ) showing hyperostosis.
The muscular system of fish has both red and white skeletal muscle. White (fast or twitch) muscle predominates and is important for anaerobic burst or sprint swimming (Roberts and Ellis 2012). Red (slow) muscle is associated with sustained aerobic swimming and has more blood supply; this muscle typically lies in a thin band under the skin along the lateral line and/or dorsal midline (Greek‐Walker and Pull 1975). Pelagic and more active fish have a higher proportion of red muscle (Greek‐Walker and Pull 1975). Drug pharmacokinetics are likely affected by muscle type although the impact is not well‐known. The scup ( Stenotomus chrysops ) has pink muscle, which has less myoglobin than red muscle, and icefish in the arctic family Channichthyidae have yellow muscle due to a lack of hemoglobin (Helfman et al. 2009).
Fish muscle and skin are generally inelastic; therefore, injection volume and depth are important considerations. Intramuscular medications are potentially more likely to cause injection site lesions than in other vertebrate classes and volumes should be small. Leakage from injection sites is also common both immediately and once fish begin to swim after injection, which is likely to alter pharmacokinetics ( Figure A1.3) (Fredholm et al. 2016).
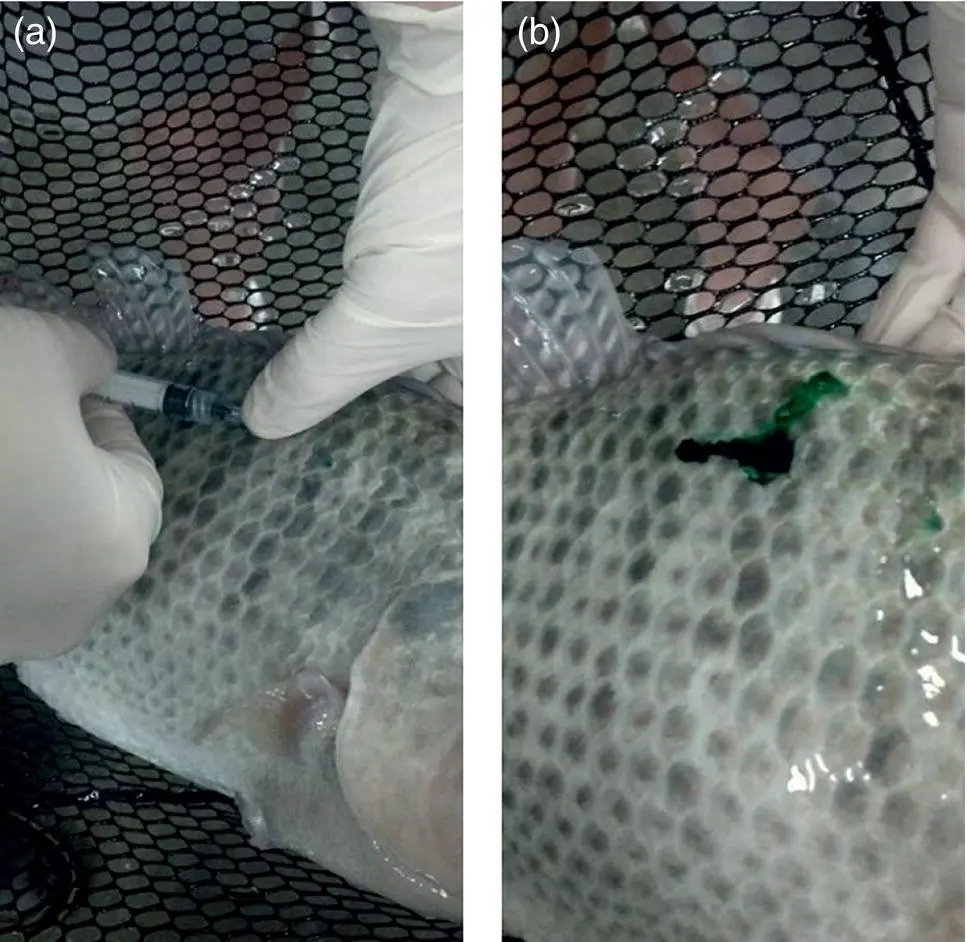
Figure A1.3 Leakage of drug with green marker following intramuscular injection in a Nile tilapia ( Oreochromis niloticus ).
Most fish are poikilothermic, with body temperature matching water temperature. A few bony fish species show regional endothermy, maintaining their body temperature above ambient, e.g. tunas ( Thunnini ), billfish ( Istiophoridae ), and one species of mackerel ( Gasterochisma melampus ). Endothermy is accomplished using retes in the brain, muscle, and viscera, and using red muscle located near the vertebral column. Endothermy improves digestion and nerve and muscle activity and is important for large predators chasing fast prey in colder waters (Block and Finnerty 1994).
Electrogeneration is possible from modified skeletal muscles in a variety of bony fish including freshwater elephantfish ( Mormyridae ), South American knifefish ( Gymnotiformes ), and electric catfish ( Malapteruridae ).
The swim (gas) bladder of bony fish shows extensive variations. Its primary function is buoyancy, but it can also be important in sound production and pressure reception (Roberts and Ellis 2012). It is absent in cartilaginous fish (chimaeras, skates, rays, sharks), some bottom‐dwelling teleosts (e.g. flounder, Pleuronectiformes ), weather loaches ( Misgurnus spp.), and some highly pelagic teleosts like tuna ( Thunnini ). The swim bladder is filled with oil or fat in some bathypelagic species, e.g. lanternfish ( Myctophidae ) and orange roughy ( Hoplostethus atlanticus ) (Blaxter and Batty 1990; Phleger 1998). The volume of the swim bladder compared to body weight is typically under 5% in saltwater fish and under 7% in freshwater fish (Blaxter and Batty 1990). The gas in the bladder is composed of carbon dioxide, oxygen, and nitrogen, but not in the same percentages as air (Helfman et al. 2009).
Two types of swim bladder exist: physostomous and physoclistous. In physostomous fish, there is a pneumatic duct that connects the swim bladder to the esophagus. The gas is maintained by swallowing air. This anatomy has its disadvantages in that foreign bodies or gavaged food may enter the swim bladder (Stoskopf 1993). Common physostomous fish are salmon and trout ( Salmonidae ), catfish ( Siluriformes ), koi and goldfish ( Cyprinidae ), and tetras ( Characidae ), although some of these species also have a rete mirabile for some gas absorption.
Physoclistous fish lack a connecting duct. Inflation occurs via blood gases diffusing along one or more rete mirabile (gas glands) or vessels in the swim bladder, typically located cranially and ventrally ( Figure A1.4) (Pelster 2011; Roberts and Ellis 2012). Physoclists include most marine teleosts, cichlids ( Cichlidae ), bass and sunfish ( Centrarchidae ). Some of these species also possess a capillary plexus caudodorsally (the oval or oval window) that helps resorb gases (Pelster 2011; Roberts and Ellis 2012).
Familiarity with normal swim bladder appearance is important to evaluate abnormalities on diagnostic imaging, endoscopy, coeliotomy, or necropsy. These may include hyperinflation, hypoinflation, displacement, and fluid or parasites. If injecting air to manage hypoinflation, a knowledge of normal volume is also important to prevent hyperinflation.
One lobe is most common. It may be U‐shaped (e.g. some pufferfish, Tetraodontidae).
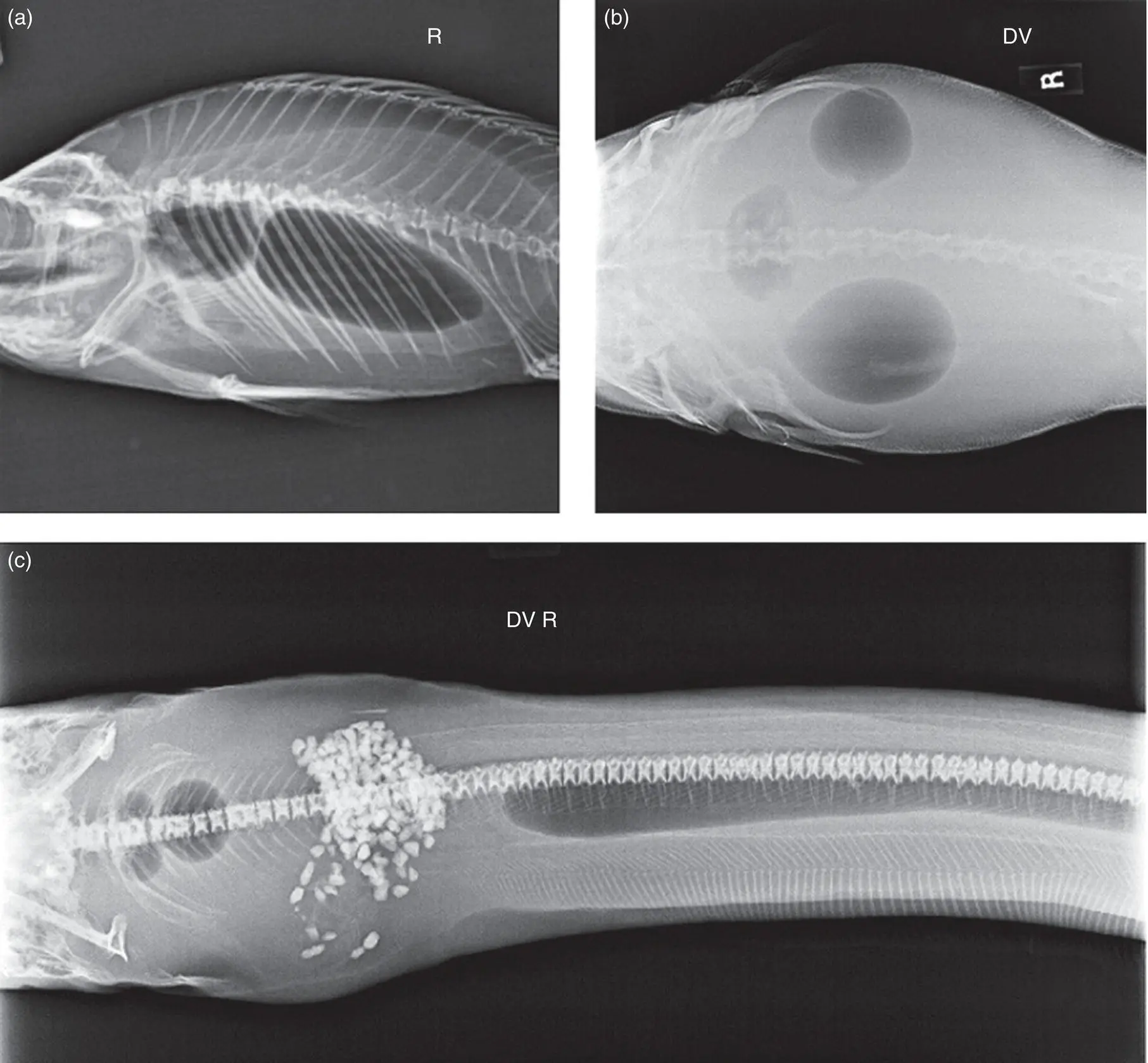
Two lobes are found in various species ( Figure A1.5a), including goldfish (Carassius auratus) and common carp and koi (Cyprinus carpio), although some goldfish breeds have lost the caudal lobe.
Three lobes are found in cod (Gadus spp.), channel catfish (Ictalurus punctatus), and some pufferfish (Arothron spp.) ( Figure A1.5b).
Extensions are common. The swim bladder may connect to the inner ear (e.g. herrings and anchovies, Clupeiformes), extend into vertebrae (e.g. freshwater angelfish, Pterophyllum spp.), or extend down the tail (e.g. electric eel, Electrophorus electricus, and arowana, Osteoglossidae) ( Figure A1.5c).
The swim bladder may be modified into lungs or lung‐like tissues (e.g. garfish, tarpon, arapaima, and lungfish, see below).
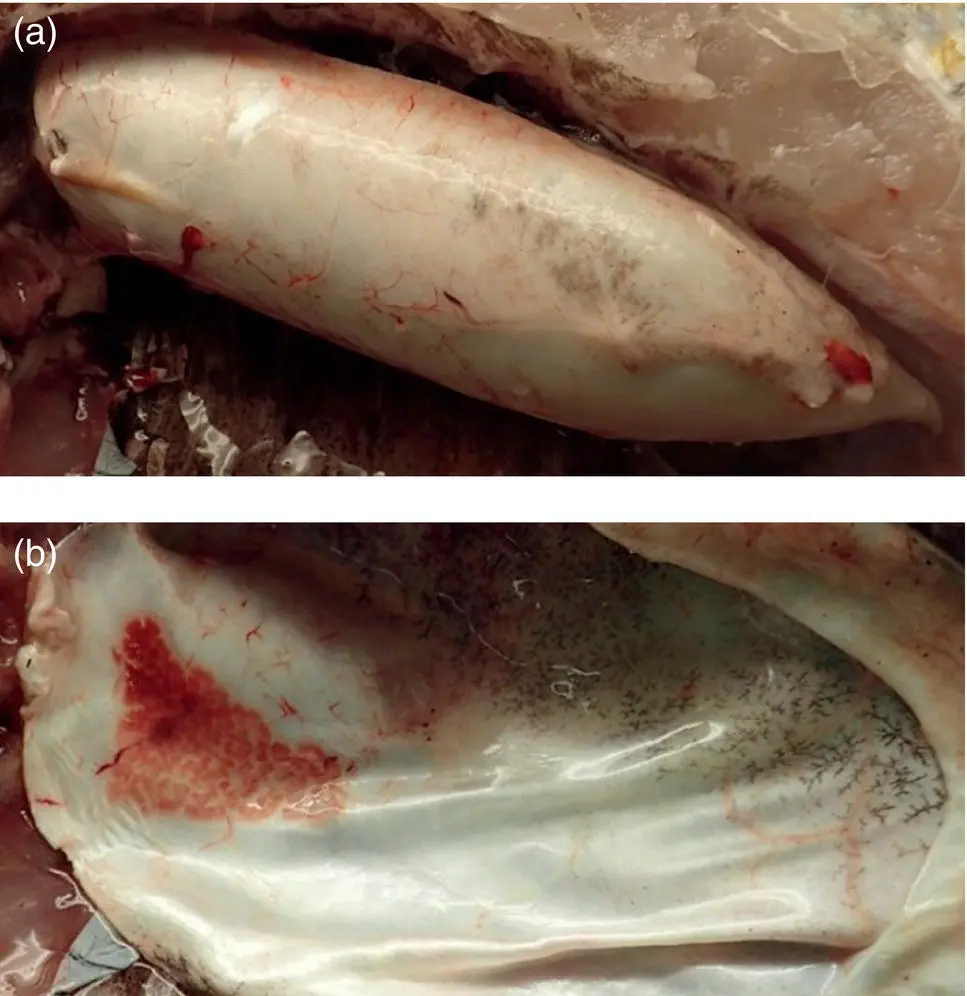
Figure A1.4 Intact (a) and incised (b) swim bladder of a bluestriped grunt ( Haemulon sciurus ) showing the rete mirabile.
Source : Image courtesy of Carlos Rodriguez, Disney’s Animals, Science and Environment.
Читать дальше
















