Diatom Gliding Motility
Здесь есть возможность читать онлайн «Diatom Gliding Motility» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Diatom Gliding Motility
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Diatom Gliding Motility: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Diatom Gliding Motility»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
This volume is the first book attempting to gather such information surrounding diatom motility into one volume focusing on this single topic. Readers will be able to gather both the current state of understanding on the potential mechanisms and ecological regulators of motility, as well as possible models and approaches used to help determine how diatoms accomplish such varied behaviors as diurnal movements, accumulation into areas of light, niche partitioning to increase species success. Given the fact that diatoms remain one of the most ecologically crucial cells in aquatic ecosystems, our hope is that this volume will act as a springboard towards future research into diatom motility and even better resolution of some of the issues in motility.
Diatom Gliding Motility — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Diatom Gliding Motility», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
13 Chapter 13Figure 13.1 Appearance of Navicula sp. diatoms under an optical microscope. Cells are about 14 µm long [13.24]. 1Figure 13.2 SEM Image of Navicula sp. in girdle band face (a) and the frustule view (b). 2Figure 13.3 Pore array and pore structure on the frustule. (a-c) Meso-porous structure in the frustule, (d) nanoporous strucuture inside mesopores. AFM mapping of pores around ridge (e) and mesopores (f). 3Figure 13.4 The bending ability of the diatom wall during the re-positioning process, scale bar is 5 μm. (a-c) The diatom indicated by the arrow attaches to the wall and the diatom on the right side is changing its orientation by rotating in situ, which the maximum bending angle is about 37 degrees in (b).Figure 13.5 Equipment for analyzing bending ability of diatoms. See text for description. (a) Experimental set up of electrode corrosion for preparing tungsten needles, (b) characterization of an as-prepared tungsten needle, (c) experiment set up of bending ability characterization inside the SEM equipped with a micromanipulator and a force sensor. 4Figure 13.6 The relationship between bending deformation and stress of frustules. The scale bar is 5 μm. (a) original position, (b) bending deformation with an angle of 26 degree, (c) forces measured with deformation.Figure 13.7 Simplifying the frustule bending into a simple cantilever beam system.Figure 13.8 Classical Edgar model. See text for description of components.Figure 13.9 Pits found in the mucilage trails. Scale bar equals 2 μm. 5Figure 13.10 Diatoms locomoting while raised at an angle of inclination. 7Figure 13.11 Cross-section view of diatom locomotion, (a) normal locomotion, (b) inclined locomotion.Figure 13.12 The same diatom crawls along the raphe (a-b) and its girdle band (c-f) surfaces. 8Figure 13.13 Diatoms crawl with the girdle band facing the substrate.Figure 13.14 The circular structures in the body of some locomoting diatom cells. (a-d) are serial captures of the same diatom while locomoting, which two circular structures were observed to move within the cell body at high-frequency vibration at the microscale. 9Figure 13.15 Obscure circular structures in locomoting diatom cells. Circular structures could be observed in some diatoms (a) but not always could be found (b) even under LSCM. 10Figure 13.16 F-actin (green) stained by FITC-Phalloidin. Red color is due to autofluorescence of the diatom chloroplasts. Scale bar equals 5 μm. 11Figure 3.17 Change of circular structures and space competition with chloroplasts of locomoting diatoms after encountering obstacles. (a-c) a diatom was approaching an obstacle, in which circular structures were clearly seen and chloroplasts was stained with red color. (d-f) Since the diatom can not move the obstacle, it chose to return and the circular structure was squeezing against the chloroplasts to get itself backward.Figure 13.18 Z-stack scanning of a diatom from bottom plane (Z axis coordinate is 1.82 μm) to top plane (Z axis coordinate is 7.60 μm), corresponding from (a) to (d).Figure 13.19 Mucilage trails under SEM, in which pits could be clearly seen in (b) and (c). Scale bars equal to 5 μm (a) and 1 μm (b, c). 12Figure 13.20 EDAX of mucilage trails and silicon substrate. 13Figure 13.21 Raman spectra of mucilage trail. SM denotes the spectra from secreted mucilage, while TM-1, TM-2, and TM-3 represent the spectra from three representative mucilage trials.Figure 13.22 Lattice map of adhesion of the mucilage trail.Figure 13.23 Topology of a piece of mucilage trail under AFM Scanning: (a) & (b) are two randomly selected sections.Figure 13.24 Typical AFM measurements in culture medium (a) and mucus (b).Figure 13.25 (a) Force curve against mucilage trails, (b) force curve against EPS.Figure 13.26 Schematic diagram of return process after contact between probe tip and wall. (a) A typical force curve against the trail mucilage, (b) a typical force curve against EPS.Figure 13.27 Schematic diagram of polymer morphology of the mucus (a) and the EPS (b).Figure 13.28 Function of the mucus.Figure 13.29 Scheme of a cycle of repeatable steps (a-e) when a diatom is locomoting to the right in VW model. Briefly, two circular structures alternately use actins as anchors and changing their positions inside the diatom thus generate forces to achieve the locomotion, please refer to the detail description in the text.Figure 13.30 The processes on the cell membrane of diatom contacting the frustule at the raphe. 16Figure 13.31 Locomotion trajectory of several diatoms. Each number represents the trail of a separate diatom.Figure 13.32 Determination of locomotion angle for each step. Positions 1, 2, and 3 represent 3 consecutive positions observed for a motile cell. When diatoms reach position 2, 15 s after position 1, the path direction is defined from the center point of the cell at each time point. After the cell moved to position 3 after another 15 s, the angle of turning from position 2 to position 3 was defined as α, in which α > 0 was defined as turning in a clockwise direction as observed.Figure 13.33 Distribution of locomotion angle.Figure 13.34 Distribution of locomotion length.Figure 13.35 Histogram of locomoting rate of diatoms on different walls and the velocity distributions.Figure 13.36 Comparison of secretion amount of diatom mucus: (a) mucus (b) EPS. Scale bar in (a) 5 μm. 18Figure 13.37 Diffusion and dissolution of diatom secretion.Figure 13.38 Comparison of simulation results (b) and measured results (a) of diatom locomotion trajectory. 19Figure 13.39 Locomotion trajectories of diatoms under different perception parameters: (a) Perception angle = 0°, perception threshold = 1, (b) Perception angle = 0°, perception threshold = 5, (c) Perception angle = 45°, perception threshold = 1, (c) Perception angle = 45°, perception threshold = 5, (e) Perception angle = 90°, perception threshold = 1, (d) Perception angle = 90°, perception threshold = 5.Figure 13.40 Simulated results of diatom adhesion from NetLogo. (a) very strong aggregation, which VMR>20, (b) strong aggregation, which 20>VMR>10, and (c) moderate aggregation, which 10>VMR>1.Figure 13.41 Comparisons between simulated and experimental values of VMR under different perception thresholds either with (a) or without (b) the introduction of cell division, using different perception angles. The two horizontal dashed lines correspond to the 95% confidence intervals from the solid line representing the VMR value determined directly from diatom cultures.Figure 13.42 Simulation results of diatom adhesion under different conditions with proliferation: (a) Perception angle = 0°, perception threshold = 1, (b) Perception angle = 0°, perception threshold = 5, (c) Perception angle = 45°, perception threshold = 1, (d) Perception angle = 45°, perception threshold = 5, (e) Perception angle = 90°, perception threshold = 1, (f) Perception angle = 90°, perception threshold = 5, (g) Perception angle = 135°, perception threshold = 1, (h) Perception angle = 135°, perception threshold = 5.Figure 13.43 The movement trend of daughter cell diatoms after cell division: (a-c) cells stayed in place; (d-f) cell migrated away after cell division. 20
14 Chapter 14Figure 14.1 A whimsical view of diatom motility, “ Cymbella , Epithemia and Licmophora ” by the late Steve Edgar [14.95].Figure 14.2 Diatoms somersault via protruding muscles (1753). The oat-animal, the diatom Craticula cuspidata [14.308], with its two “muscles” protruding from the two ends was described having movements [14.20] analogous to somersaulting [14.108] (reprinted with permission of and designed by Freepik Company; Portrait of naturalist Henry Baker (1698-1774) [14.415] under the Creative Commons Attribution 4.0 International license).Figure 14.3 Andrew Pritchard, naturalist and microscopist [14.408] (1804-1882). 1843 daguerreotype by A.F.J. Claudet [14.351] (public domain image).Figure 14.4 Jean Baptiste Bory de Saint-Vincent, naturalist (1778-1846) [14.406] (public domain image).Figure 14.5 Pierre Jean François Turpin, artist and botanist (1775-1840) [14.404] [14.407] [14.410] (reprinted under the Creative Commons Attribution-Share Alike 4.0 International license).Figure 14.6 The waving fibril model for diatom motility of Robert Jarosch (1962). “Figures 1 to 4 illustrate the relation between the direction of movement of the submicroscopic transverse waves in the hypothetical protoplasmic fibrils and the consequent gliding movements. Figure 1. A single free fibril: the waves running in one direction (W) cause the shifting of the fibril in the opposite direction (P). Figure 2. Longitudinal section through a Chara cell: the undulations (W) of the fibrils, which are fixed to the cell wall (C), cause the shifting of the inner layer of protoplasm (P). Figure 3. Longitudinal section through a gliding organism: (W) direction of waves in extramembranous surface-fixed fibrils; (P) direction of gliding; (M) mucilage; (S) substrate. Figure 4. Direction of waves in extramembranous fibrils during a contraction of Bacillaria paradoxa : (A) an individual cell; (S) substrate” [14.177] (reprinted with permission of Robert Jarosch. Recent photo of Robert Jarosch courtesy of Angelika Jarosch and Ilsa Foissner).Figure 14.7 Diatoms crawl like snails (1838). The motile pennate diatom Epithemia smithii [14.352] as a snail, as described by Christian Gottfried Ehrenberg (1795-1876) in 1838 [14.98]. Right: Christian Gottfried Ehrenberg, naturalist, painted by Eduard Radke ca. 1855 [14.410] (public domain image). Left: His critic, Carl Nägeli, botanist (1817-1891)[14.409] (public domain image).Figure 14.8 The diatom motor is a jet engine (1849). Top: The diatom Gomphonema acuminatum [14.190] [14.348] (scale bar 10 µm) as a jet engine [14.69] (open access; not subject to copyright restriction; the latter reprinted with permission under a GNU Free Documentation License, Version 1.2). Middle left: Aeronautics engineer Francis Herbert Wenham (1824-1908) in 1866 [14.414] (public domain image). Middle right: Lothar Hofmeister, botanist and cell physiologist (1910-1977) [14.195] (reprinted with kind permission of P. Amand Kraml, Director, Observatory of Kremsmünster). Bottom: Diatomist Hamilton Lanphere Smith (1819-1903) [14.350].Figure 14.9 (a) Lesley Ann Edgar, diatomist (1955-2006) [14.65] (image reprinted with permission of Taylor & Francis). (b) Jeremy Pickett-Heaps in 1990 [14.281].Figure 14.10 Jabez Hogg (1817-1899), ophthalmic surgeon [14.260] (reprinted with kind permission of Andrew Tucker, Assistant Curator, Museum of Freemasonry, ©Museum of Freemasonry, London, UK).Figure 14.11 Rowing diatoms (1855). Top: “Quadremes were powerful warships with two banks of oars and multiple rowers per oar” [14.277]. Middle: The diatom oars seen by Jabez Hogg (1855) [14.161]. Next: Mucilage protruding from a raphe of Navicula cuspidata in an SEM of a critical point dried cell [14.90] (reprinted with permission of Springer Nature), scale bars 10 and 1 µm, contrast enhanced by histogram equalization [14.301]. Bottom left: “ Navicula confervacea :… Raphe with organic material: valve without marginal spines,” scale bar = 1 µm [14.307] (reprinted with permission of John Wiley and Sons). Bottom right: Scanning electron micrograph showing a single file row of secreted fibrils in purported P innularia viridis . Scale bar 0.5 µm. (From [14.154] with permission of John Wiley and Sons). Note that the fibrils are not adjacent to one another, suggesting that they come out of the raphe below individually. See also [14.156].Figure 14.12 Bacteriologist Émile Pierre-Marie van Ermengem (1851-1932) [14.412] (public domain image).Figure 14.13 “Transverse section showing general cellular organization in the region of the pyrenoid [py]. Chloroplast (cp), region of the girdle (gi), intra-pyrenoid lamellae (1), mitochondria (m), nucleus (n), nucleolus (no), raphe (r)” [14.359] (reprinted with permission of Springer Nature). Note a single fibril in the top raphe extending inside past a gap to the cell membrane, below which is a pair of microfilament bundles. The distance between opposite raphes in Cocconeis diminuta is 3 µm [14.359]. Similar gaps have been imaged also by TEM [14.80].Figure 14.14 Left: Max Schultze, microscopic anatomist (1825-1874) [14.405] [14.417] (public domain image). Right: Theodor Wilhelm Engelmann (1843-1909), botanist, physiologist, and microbiologist [14.424] (public domain image).Figure 14.15 Diatoms have protoplasmic tank treads (1865). Top left: The diatom as a double tank tread in girdle view. In order to ensure independence of the two raphes, two tank treads would be necessary. (Adapted from [14.286]. Bottom left: The double tank tread model for diatom motility (1893): “I interpret these phenomena in such a way that a current of cytoplasm is driven through the pole cleft of the anterior end node into the outer cleft of the raphe, there is shifted towards the center and flows back into the cell interior through the outer central node channel” [14.252]. A similar diagram is shown in [14.44]. Right: Georg Ferdinand Otto Müller (1837-1917) [14.419] (public domain image) published a series of 8 papers on diatom motility [14.251] [14.252] [14.253] [14.254] [14.255] [14.256] [14.257] [14.258] [14.387].Figure 14.16 Left: In 2015: “We propose a model [for gliding of Flavobacterium johnsoniae ] in which a pinion, connected to a rotary motor, drives a rack (a tread) that moves along a spiral track fixed to the rigid framework of the cell wall. SprB [a cell-surface adhesin], carried by the tread, adsorbs to the substratum and causes the cell to glide…. Tethered cells pinwheel around a fixed axis, suggesting that a rotary motor that generates high torque is a part of the gliding machinery [14.334]…. If 90 nm is the radius of a pinion rotating 3Hz (the maximum speed of rotation when a cell is tethered) then that pinion can drive a rack (a tread carrying adhesins) at 1.5 µm/s, which is the speed that cells glide. This suggests that nature has not only invented the wheel, it also has invented the likes of a microscopic snowmobile…. A model of the gliding machinery. (a) A cross-sectional view of a cell with a rotary gliding motor (blue), a mobile tread (green), a stationary track (red), and an adhesin (magenta). The rotary motor and the track are anchored to the peptidoglycan (PG), and the track is wound spirally around the cell. The rotary motor drives a pinion that engages a mobile tread (rack) that slides along the track. The adhesin, SprB, is attached to the tread and moves with it. The dimension d is the distance between the axis of rotation of the motor and the center of the track, and r is the radius of the pinion. (b) A side view of a cell with a rotary motor powering the motion of a tread carrying SprB” [14.332] [14.333]. O.M. = outer membrane, C.M. = inner cell membrane. Right: A rack and pinion.Figure 14.17 Model for a diatom that moves but leaves no trail [14.75]. Of course, diatoms also lay down the trail.Figure 14.18 Diatoms as the Flame of Life: Capillarity (1883). (a) A candle draws up molten wax by capillarity into the wick, where it burns off along the length of the wick, emitting light as it enters the gaseous phase and ignites. For a diatom the raphe is a hydrophobic wick drawing hydrophobic raphe fluid (raphan) from inside the diatom. As raphan consists of polysaccharide fibers, particles can stick to them as they exit and move along the raphe, carrying the particles along the raphe. If the raphe fluid adheres to a large object or a substrate, the diatom moves in the opposite direction [14.122]. As the raphan comes into contact with water, it hydrates, can no longer wet the raphe, and exits to the medium. The diatom trail is analogous to the flame of a candle. (b) “The capillarity model for diatom gliding locomotion, as originally conceived in [14.122]. A schematic ‘longitudinal slice’ of a single raphe is shown as if the raphe went straight through the silica valve. (It is actually hooked in cross section.) The crystalloid bodies empty their fibrillar, mucopolysaccharide contents into the raphe via exocytosis. The role of the microfilaments was speculated to be control of the distribution of the exocytosis along the raphe in an unspecified manner. The directionality of the motion could come either from an asymmetric distribution of release of mucopolysaccharide along the raphe, or, as shown here, from a postulated difference in rate of hydration of the mucopolysaccharide between the leading and trailing pores. The hydration of the mucopolysaccharide may also permit it to stick to the substratum, as indicated. This results in motion in the direction shown. The released, fully hydrated mucopolysaccharide stays attached to the substratum as trail material. While capillarity fills the raphe with the mucopolysaccharide, its hydration removes it and provides the driving force. (Hydrated mucopolysaccharide no longer wets the walls of the raphe, suggested to consist of a hydrophobic lipid layer by [14.92]” [14.117] (reprinted with permission of Elsevier). The sketch has been rotated and aligned to correspond to the candle. If the substrate were replaced by small particles, they would rise upwards just as the liquid wax does in a candle. The sketch has been modified to show how the microfilament bundles may permit access of the crystalloid bodies [14.79] at one end of the raphe but not the other, by sliding along their length. Note: “We have electron micrographic evidence that indeed these vesicles are secreted into the raphe canal” [14.47]. In this model, the motile pennate diatom is the flame of life.Figure 14.19 Bellowing diatoms (1887). Left: Stauroneis baileyi , shown in girdle view, was found to bellow out, with the distance between a and b increasing, when moving in the direction of the arrow [14.342]. Right: A selfcompressing bellows, on blowing air out, would move in the same direction [14.106] (public domain image).Figure 14.20 Top: In 1896: Cross sections of the pennate diatom Pinnularia major from the middle to the end of the cell showing how the raphe is a slit through the whole valve [14.205]. Bottom: Robert Lauterborn in 1928 [14.239] (reprinted with permission of Elsevier).Figure 14.21 Jelly powered jet skiing diatoms (1896). In 1892: “… Pinnularia nobilis in motion in the view on the girdle side. n the nucleus, c the centrosome, x the peculiar double threads in the plasma, a the inflow to the node (k) of the anterior raphe, b the gelatin thread that shoots out to the rear, which has rolled up at the right end at the end. The arrows indicate the direction of movement of the diatom, the inflow and the thread” (translated from [14.44]). Otto Bütschli (1848-1920) [14.418] attributed the diatom’s motion to pulling, pushing and recoil of the threads, rather than the inward flow he thought he observed. (Photograph of Bütschli by Max Kögel [14.191] with permission of Universitätsbibliothek Heidelberg under the Creative Commons Attribution-Share Alike 4.0 International license).Figure 14.22 Bubble powered diatoms (1905). Left: The bubbly motile diatom. Middle: Nitzschia acicularis [14.368]. Right: View of a portion of the Chemical Laboratory at the Mt. Prospect Laboratory of the Brooklyn Water Works, next door to the Biological Laboratory, where Daniel D. Jackson [14.80] worked [14.403].Figure 14.23 Diatoms win: “I have no new theory to offer and see no reason to use those already abandoned” (1940) [14.230]. Left: Medical botanist Pierre Martens (1895-1981) in 1950 [14.73]. Right: Martens’ depiction of Otto Müller’s flow model: “Connective view of a valve, showing the channeling at the level of the median nodule and the paths attributed to the propellant currents. The median nodule is drawn (after Müller), in an orientation which does not show the outlet of the ‘external slit’ outside; the internal structure of the polar nodules is not shown. The upper arrow indicates the direction of locomotion…. Abbreviations: np = polar nodule; nc = central nodule; fe = external slot of the raphe; fi = internal slot; rc = groove of the central nodule, joining the two internal slots of the same valve; cu = small channel joining the external and internal slits” 13[14.230].Figure 14.24 Is diatom motility a special case of cytoplasmic streaming? (1943). Left: Early model of what we now call a motor protein invoked for cytoplasmic streaming. 1) Protein molecule in extended form. Bond at anterior end of molecule. (2) Protein molecule in contracted form. (3) Shifting of bond from anterior to posterior end. (4) Protein molecule in expanded form…. Let us say that the molecules which form bonds in this way so as to contribute to the motion are in phase and those which may form bonds so as to oppose the motion are out of phase. It is clear that streaming can occur only when the number of molecules in phase exceeds those out of phase. This cannot happen by chance alone and we must therefore postulate that some mechanism is present which sets the majority of stream proteins in phase. This mechanism must of course be intimately related to the mechanism responsible for the reversal of streaming” [14.222] (reprinted with permission of the American Philosophical Society). Right: Motion of an inchworm caterpillar [14.287] (reprinted with permission of Elsevier).Figure 14.25 “Row, row, row your boat. The molecular motors in the myosin heads pull the myosin filaments (blue) over the actin filaments” [14.157]. This version from [14.46] (reprinted with permission of Cambridge University Press). Cf. Figure 14.11.Figure 14.26 Model for an amoeba (1999) [14.10] (reprinted with permission of Elsevier).Figure 14.27 In 2004: “A schematic representation of a likely mechanism underlying the fluctuation enhancement of an actin filament, induced by Chara myosin molecules. (a) Disequilibrating state: A myosin head generating the sliding force influences the kinetics of the neighboring heads along the actin filament, in which either the pushing or the pulling force is generated. (b) Coordinating state: These activated heads develop a coordination among themselves along the filament. Coordination in one place then induces a disequilibrium out of coordination in the neighborhood. Equilibration and desequilibration thus reiterate” [14.144] (reprinted with permission of Elsevier).Figure 14.28 “Schematic picture of the delivery of different cell wall components to the plant plasma membrane…. Question marks indicate that no direct experimental evidence for the localisation of the component is available…. The vesicles containing the CESAs [cellulose synthase proteins] are transported to the plasma membrane with the help of actin cables” [14.111] (reprinted with permission of Elsevier). A myosin XI such as in the class of those involved in cytoplasmic streaming functions in cellulose exocytosis [14.441].Figure 14.29 Top left: “L.S. [lateral section] in valve view ( Navicula cuspidata ) showing detail of microfilamentous bundles and associated vesicles; some vesicles appear attached to the bundles (arrow). Scale = 1 µm” [14.93] (reprinted with permission of Elsevier). These are presumed to carry raphe fibrils (raphan). Top right: An F-actin = filamentous actin = microfilament can carry secretory vesicles to the cell membrane [14.337]. In this depiction, transport of vesicles can be either by direct attachment to myosin or indirectly via hydrodynamic flow [14.42] (reprinted with permission of John Wiley & Sons). The attachment of myosin to a vesicle may involve “membrane-anchored core myosin receptors, possibly aided by adaptors” [14.279]. Myosin XI/vesicle attachments are transient, lasting only a few seconds [14.30]. Middle left: An explicit model for binding of myosin to a membrane, in this case the outer (ONM) and inner (INM) nuclear membranes [14.442] (reprinted with kind permission of Iris Meier). Bottom: “The microfilament model for diatom gliding locomotion, as conceived by Edgar & Pickett-Heaps (1984) [14.93], and adapted from their Figures 39, 40 and 41. All features are the same as in [Figure 14.18], except that: (a) the mucopolysaccharide fibrils are assumed to be attached to the microfilament bundles through the plasmalemma, which has no effect because of its fluid nature, via “the lining of the vesicles [crystalloid bodies] in which the strands were originally synthesized”; (b) hydration is assumed to occur along the whole length of each mucopolysaccharide fibril while it is still within the raphe; (c) the fibrils are assumed to swell and elongate as they come out of the crystalloid bodies; (d) a mechanism is needed by which “the mucilage strands are broken free from the plasmalemma on reaching the apical raphe ending [trailing pore].” The microfilament bundle is presumed to provide the motive force. Reversal of the direction of motion is suggested to occur as follows: “Since... there are two bundles of filaments..., perhaps the actin filaments in each bundle are oriented in one direction, and the polarities in the two bundles of filaments are opposite. If this were so, bidirectional movement could be envisaged as occurring as...the raphe adhesive is moved by some controlled activation of or coupling with the actin bundles alternately.” Only one microfilament bundle is shown. The other, oppositely oriented, would be placed behind this one. Thus, two rows of fibrils are possibly present in a raphe simultaneously. The second row would be behind the one shown” [14.117] (reprinted with permission of Elsevier).Figure 14.30 Diatom adhesion as a sliding toilet plunger (1966). Left: “Suction cup with handle, used to study suction-seal-substratum relationships” [14.79] (reprinted with permission of Springer Nature). Right: Ryan W. Drum in 2003.Figure 14.31 Diatom as a monorail that lays its own track (1967). Top: In 1967: “Either of the mechanisms indicated…could keep the trail against the raphe and explain adhesion forces. In the…osmotic… hypothesis…(a), the trail T is held in position by low pressure in the water W in the raphe slit. The pressure would be kept low by osmosis across the cell membrane. Alternatively,…the interfacial tension hypothesis… (b) shows the raphe filled with a liquid L immiscible with water. Interfacial tension at I would then keep the trail in place…. Schematic raphe cross-sections. Substrate stippled. I , water-liquid interface; L , liquid; T , trail; W , water at low pressure being sucked into the diatom by osmosis” [14.142] (reprinted with permission of Taylor & Francis). Middle right: A monorail train gripping its track [14.309] (public domain image). If diatoms do indeed lay down their own track, we have much to learn from them [14.430]. Bottom left: Margaret A. Harper and John F. Harper, wedding photo taken at Willesden Parish Church, NW London, UK, 29 August 1964. Bottom right: Margaret A. Harper and John F. Harper, at present, with their permission.Figure 14.32 “Four locomotion theories [as of 1977], represented on schematic sections of pennate diatoms in their apical planes. Trail precursor is shown by coarse stipple, trail by fine stipple. (a) Jarosch (1962) [14.177]. Mucilage secreted at pores a and c being driven by undulating actin filaments connected to the protoplast. (b) Hopkins & Drum (1966) [14.164]. Trail secreted through entire lengths of raphes ab and cd and expanding on leaving the raphe system. (c) Harper & Harper (1967) [14.142]. Motion due to trail secretion from pore a. Secretion from upper pores e and h forming lump at l and particle p being carried forward. (d) Gordon & Drum (1970) [14.122]. Capillary flow of a liquid along raphes ab and cd, conversion to trail at pores b and d” [14.141] (reprinted with permission of John Wiley and Sons). Note that the central nodule is lacking in diatoms such as Bacillaria [14.439], which either reduces the complexity from four pathways, as in Liebsch (1929) [14.218], or indicates that the central nodule does not play the role suggested here.Figure 14.33 The diatom as a “compressed air” Coanda Effect gliding vehicle (1967). A wing profile assisted by compressed air coming out of the plenum chambers, with the air flowing close to the surface due to the Coanda effect [14.22] (reprinted with kind permission of Stephen D. Prior, superimposed by diatom Cymbella cistula [14.244] with permission of the Muséum national d’Histoire naturelle under a Creative Commons license). Of course, the raphes should be rotated 90° for a proper match.Figure 14.34 The electrokinetic diatom (1974). Depiction of ionic currents that break the symmetry of the spherical Fucus egg [14.172] (reprinted with permission of Elsevier). Such currents, if they occur in pennate diatoms, could bias the flow of charged molecules in the raphe or the cell membrane just within the raphe or the microfilaments adjacent to the raphe. Reversal of the field would then be predicted when the diatom reverses direction.Figure 14.35 Discovery of the “crystalloid bodies” and their relationship to the raphe. Left: “1. A three-dimensional view of the silica structure of a diatom. The raphe system (RS) is composed of a central pore (CP) and a terminal pore (TP) with an outer groove of the raphe fissure (R). Continuation grooves (CG) and a probable anterior pore (AP) are shown. 2. A raphe plane section of a diatom moving upon a plane surface (GS) showing the four pores as in #1. The cytoplasm contains a fibrillar bundle (FB) and crystalloid bodies (C) containing minute fibrils (F). These are found in the diatom raphe system and in the diatom trail. The point of locomotor-adhesion contact (LAC) is indicated. 3. A raphe plane section of a diatom moving upon particles (PS). In this case, locomotor-adhesion contact (LAC) can involve any point along the raphe system” [14.164] (reprinted with permission of Taylor & Francis). Right: “Transverse cross-section of a raphe fissure and adjacent cytoplasm of a diatom moving over a particulate substratum; the locomotor adhesion seal (arrow), firmly attached to particles, is being pushed along raphe by streaming directed against that seal; the fibrillar bundle (F) lies next to the raphe (R); longitudinal and transverse sections of crystalloid bodies (CB) are also shown” [14.79] (reprinted with permission of Springer Nature). The fibrillar bundles were later identified as similar to smooth muscle and presumed to contract, pushing the crystalloid bodies and moving the diatom [14.173].Figure 14.36 The diatom clothes line or railroad track (1980). Left: Clothespin line model for diatom motility, amalgamated from [14.11] [14.427] (public domain image). Top to bottom: microfilament bundle, myosins, clothespins are membrane bound raphan synthase carrying raphan inside a raphe, where raphan is a polysaccharide raphe fibril. Top right: “Model for the organization of the motor apparatus in diatoms. Adhesive mucilage secreted by the diatom adheres to the substrate and binds to as yet undefined transmembrane components. The cytoplasmic domain of the membrane-associated complex is linked to a diatom myosin which actively translocates the membrane complex and attached mucilage rearward along a track of cortical actin filaments, leading to forward gliding of the diatom” [14.146] (reprinted with permission of Elsevier). This is the generally assumed model of Lesley Edgar and Jeremy Pickett-Heaps, though they also allowed for an indirect coupling of the myosin to the raphe fibrils [14.93]. Bottom Right: Similar model of Rick Wetherbee et al . (1998): “Diagram suggesting how the adhesion complex would look in a raphid diatom. Note that only the actin [microfilament] has been described for certain. There has been no attempt to illustrate where components responsible for motility (e.g., a motor) might be located” [14.401] (reprinted with permission of John Wiley & Sons). The actin-associated proteins would presumably be myosins, and the transmembrane proteins would be raphan synthase.Figure 14.37 Diatom ion cyclotron resonance (1987). A take on diatoms and cyclotrons [14.207] (public domain image). Accelerating diatoms are Cymbella cistula [14.244] (reprinted with permission of the Muséum national d’Histoire naturelle under a Creative Commons license).Figure 14.38 Diatoms do internal treadmilling (1998). Left: In treadmilling of microfilaments, actin monomers are added at one end and removed at the other end, resulting in a constant length, with motion [14.45] (reprinted with permission of Springer Nature). Upper right: Molecular motors may be involved in regulating the length of a treadmilling microfilament: “A treadmilling filament is described by a lattice of dynamic length. Motors are represented as particles that occupy the sites. At one end sites are added at a rate of α. At the opposing end, empty (occupied) sites are removed at a rate of β 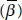 . Particles attach to empty sites at a rate of ω and detach from the lattice at a rate of
. Particles attach to empty sites at a rate of ω and detach from the lattice at a rate of 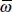 . Particles hop to adjacent free sites in the direction of the shrinking end at a rate of γ” [14.100] (reprinted with permission of the American Physical Society Publishing). Lower right: “A model showing how myosin driven actin sliding with the combination of tethering proteins can potentially drive ER [endoplasmic reticulum] and Golgi mobility. Myosins are shown linking actin filaments within a bundle and are responsible for filament sliding” [14.234] (reprinted with kind permission of Joseph F. McKenna). Similar sliding might be occurring in the microfilament bundles adjacent to raphes.Figure 14.39 Flow along a line, such as a raphe, induces a circulating flow of the adjacent fluid, by analogy to the early neural plate in a chicken [14.402] (reprinted with permission of Springer Nature).Figure 14.40 Rough schematic for a diatom robot (diatombot), a neutrally buoyant, remotely controlled robot that simulates motion of the raphe fluid using bungee cords moving on pairs of motorized pulleys to test the possibility that motile diatoms can swim. The outside portions of the bungee cords lie in grooves, allowing them to make contact with the water outside. Particles in the water could show up in any induced water flows.Figure 14.41 Surface treadmilling, swimming and snorkeling diatoms (2007). A snorkeling pennate diatom.Figure 14.42 Acoustic streaming: the diatom as vibrator or jack hammer (2010). Perhaps a vibrating diatom can smash its way through sand, like a jack hammer removes concrete [14.306] (reprinted with kind permission of Robert Bosch Tool Corporation). The diatom is Lyrella esul [14.338] (reprinted with kind permission of David A. Siqueiros Beltrones).Figure 14.43 Propulsion of diatoms via many small explosions (2020). Nuclear propulsion via hundreds of “small” nuclear bombs: Project Orion (conceived by Stanislaw Ulam, 1946), an analog of diatom propulsion via explosive hydration of raphe fibrils. Top left: artist’s conception [14.420]. Top right: Principle [14.315]: The “Pulse unit injection” would correspond to a raphe carrying raphe fibrils that explosively hydrate on being exposed to water. Middle right: A space faring diatom [14.119] [14.124] Lyrella esul propelled by explosions [14.134]; Lyrella esul [14.338] with kind permission of David A. Siqueiros Beltrones. Bottom: Project Orion configuration [14.420], all others from NASA, in public domain).Figure 14.44 Diatoms walk like geckos (2019). Was the gecko inspired by the diatom, or vice versa? (Gecko [14.206] . Pseudoraphid diatom Podocystis adriatica [14.139] with permission of Paul Hargraves and the New England Botanical Club).Figure 14.45 Intraprotein pores in hyaluronan synthase and cellulose synthase. Top left: “A model for interaction of the HAS [hyaluronan synthase] tandem-motif region with HA [hyaluronan, the #s refer to membrane domains]. The… growing HA-UDP chain, containing alternating GlcNAcβ1,4 (blue squares) and GlcUAβ1,3 (blue-white diamonds) attached to UDP [uridine diphosphate] (red inverted triangle) at the reducing end. Preliminary results indicate that the nonreducing end contains a chitin oligomer cap with four GlcNAc residues, which is the primer on which HA synthesis is initiated” [14.18] (reprinted with permission of Oxford University Press). Top right: AFM experiment suggesting that raphe fibrils are multistranded [14.153] (like cellulose) (reprinted with permission of Elsevier). Bottom: “Updated models of plant CESAs [cellulose synthases] and CSCs [cellulose synthesis complexes]. (a) A computational model of a plant CESA catalytic domain with P-CR and CSR regions (light grey). The glucan chain (purple) is from the homologous Rhodobacter structure. The location of the transmembrane helices (TMH) is represented with grey boxes. (b) Three CESAs, encoded by three different genes, may interact to form a trimeric particle, which in turn may assemble into a hexameric rosette, depicted in (c). The glucan chains are represented in red. (Image (a) is adapted from [14.339] and is courtesy of Jonathan Davis)…. the intra-protein tunnel [pore]… provides a low-energy pathway for translocating the growing glucan chain to the external membrane surface from the cytoplasmic side, where the catalytic site transfers a glucose residue from UDP-glucose to the reducing end of the glucan” [14.62] (reprinted with permission of Elsevier).Figure 14.46 Left: “Stages in secretion at the raphe (hypothetical). (a) Polysaccharide fibrils are discharged from a vesicle into the raphe at the central pore. The preferred secretory sites are at both central and apical (not shown) raphe endings; (b) Secreted fibrils experience hydration and begin to swell and elongate. A second vesicle is docked at the plasmalemma and primed for exocytosis. (c) After considerable swelling the mucilaginous strands project through the raphe; their proximal ends are attached to the plasmalemma and their distal ends are free to make contact with a substratum; (d) Strands, which were produced at the raphe ending, have moved along the raphe but still occupy the slit and maintain attachment to the plasmalemma. Microfilaments act as a barrier preventing vesicle discharge here (not evident in all cells)” [14.93] (reprinted with permission of Elsevier). Note that the hydration of the raphe fibrils does not likely occur within the raphe, as shown, because the walls of the raphe are hydrophobic [14.92] [14.93] [14.122]. On the contrary, hydration at the external tip would generate a force pulling the fibrils out of the raphe. Right: The silica lining the raphe differs from that of the valve [14.66] (reprinted with permission of John Wiley & Sons).Figure 14.47 A general model for noncellulosic polysaccharide biosynthesis [14.362] (reprinted with permission of Oxford University Press).Figure 14.48 “TEM of thin sections of Amphora veneta . 26: T. S. [thin section] through a perinuclear dictyosome [Golgi]. The dictyosome is composed of 8 cisternae (ct) which are blebbing fibrous (v 1) and smaller granular (v 2) vesicles (× 44,000). 27: Oblique L. S. through the central region. Irregularly shaped fibrous (v 1) and smaller granular vesicles (v 2) are apparent (× 32,400). 28: T. S. part of cell to show frustule-cell-membrane interface (fcm). Large numbers of vesicles (v 4) are present outside the cell membrane (cm) but within the silica wall (sw) (× 33,000). 29: T. S. showing fibrous material (fm) extruding from a raphe fissure (rf) (× 22,400)” [14.70] (reprinted with permission of Springer Nature). Note: “The largest [vesicles] (27), were derived from the mature dictyosome [Golgi] cisternae, and were often irregular in outline whilst containing fibrillar material. These vesicles were widely distributed throughout the cytoplasm and the contents were similar in morphology to the extracellular mucilaginous material (29).”Figure 14.49 “Four possible configurations of the microfilament bundles, assuming that they are capable of sliding a short distance relative to the silica raphe. Only the ends of the raphe are shown here, represented by two pores. The view is from the inside of the cell, looking out. The placement of the raphe pores on opposite sides of the apical axis [14.34] is a common feature of raphid diatoms. In this scheme, the microfilament bundles block access of the crystalloid bodies to the raphe along their whole length. In the capillarity model, such blockage would explain the direction and reversal of motion, as indicated on the right” [14.117]. (Reprinted with permission of Elsevier).Figure 14.50 Top: Swirling, draining patterns on a bubble film draining downwards [14.250] (reprinted with kind permission of Michael Reese Much FRMS). Bottom: One frame from a movie showing how dynamic these patterns are [14.288]. The interference colors are due to varying thickness. Note that the flows organize themselves along narrow lines which can draw in material from a wide area. This could be analogous to a diatom’s raphe drawing the liquid cell membrane towards it, bearing proteins (raphan synthase) ready to secrete their polysaccharide raphe fibrils (raphan) upon reaching the raphe.Figure 14.51 Surfing diatoms, Achnanthes and Podocystis [14.95].Figure 14.52 Membrane surfing: A new working hypothesis for the diatom motor (2020). Surfing working model for diatom motility. Raphan synthase is a hypothesized membrane protein that is functional either in the cell membrane, or more likely, in vesicles (crystalloid bodies) into which it deposits raphan, the presumed polysaccharide constituting the raphe fibrils seen in raphes. The whole cell membrane flows, bringing the raphan synthases or the crystalloid bodies, represented as miniatures of Figure 14.51, to the raphe. Myosin motor molecules move along the microfilament bundles (only one shown) hydrodynamically inducing flow of the cell membrane by carrying the vesicles over the raphe. As the vesicles fuse with the cell membrane, they dump their hydrophobic contents into the raphe, where the hydrophobic raphan is shown as short, vertical lines. As the raphe lining is hydrophobic, they fill the raphe via capillarity. Those that come in contact with a substrate, such as the chondrite [14.125] shown here, hydrate, swell, and stick to it. As these hydrated raphan molecules exit, more hydrophobic raphan molecules fill in the raphe, causing a net flow of the anhydrous raphan within the raphe, shown by the small arrow. The result is that the diatom moves relative to the chondrite in the opposite direction, shown by the large arrow. On a flat substrate, the swollen raphan fibrils are left behind as the diatom trail. The flow of the cell membrane determines the direction in which the diatom moves relative to its substrate. Capillarity provides the tremendous force. “On Earth, capillary forces have to fight gravity. But in space, the only resistance is the viscosity of the liquid, which slows the flow but cannot stop it” [14.240]. At the size scale of the diatom raphe, gravity has negligible effect, so the slogan applies.Figure A14.1 Could squeezing out of raphe fluid cause diatom motility? Photo of caulking gun [A.14.4] superimposed with a Cymatopleura diatom [A.14.1] under the Creative Commons Attribution License (CC BY 4.0).
. Particles hop to adjacent free sites in the direction of the shrinking end at a rate of γ” [14.100] (reprinted with permission of the American Physical Society Publishing). Lower right: “A model showing how myosin driven actin sliding with the combination of tethering proteins can potentially drive ER [endoplasmic reticulum] and Golgi mobility. Myosins are shown linking actin filaments within a bundle and are responsible for filament sliding” [14.234] (reprinted with kind permission of Joseph F. McKenna). Similar sliding might be occurring in the microfilament bundles adjacent to raphes.Figure 14.39 Flow along a line, such as a raphe, induces a circulating flow of the adjacent fluid, by analogy to the early neural plate in a chicken [14.402] (reprinted with permission of Springer Nature).Figure 14.40 Rough schematic for a diatom robot (diatombot), a neutrally buoyant, remotely controlled robot that simulates motion of the raphe fluid using bungee cords moving on pairs of motorized pulleys to test the possibility that motile diatoms can swim. The outside portions of the bungee cords lie in grooves, allowing them to make contact with the water outside. Particles in the water could show up in any induced water flows.Figure 14.41 Surface treadmilling, swimming and snorkeling diatoms (2007). A snorkeling pennate diatom.Figure 14.42 Acoustic streaming: the diatom as vibrator or jack hammer (2010). Perhaps a vibrating diatom can smash its way through sand, like a jack hammer removes concrete [14.306] (reprinted with kind permission of Robert Bosch Tool Corporation). The diatom is Lyrella esul [14.338] (reprinted with kind permission of David A. Siqueiros Beltrones).Figure 14.43 Propulsion of diatoms via many small explosions (2020). Nuclear propulsion via hundreds of “small” nuclear bombs: Project Orion (conceived by Stanislaw Ulam, 1946), an analog of diatom propulsion via explosive hydration of raphe fibrils. Top left: artist’s conception [14.420]. Top right: Principle [14.315]: The “Pulse unit injection” would correspond to a raphe carrying raphe fibrils that explosively hydrate on being exposed to water. Middle right: A space faring diatom [14.119] [14.124] Lyrella esul propelled by explosions [14.134]; Lyrella esul [14.338] with kind permission of David A. Siqueiros Beltrones. Bottom: Project Orion configuration [14.420], all others from NASA, in public domain).Figure 14.44 Diatoms walk like geckos (2019). Was the gecko inspired by the diatom, or vice versa? (Gecko [14.206] . Pseudoraphid diatom Podocystis adriatica [14.139] with permission of Paul Hargraves and the New England Botanical Club).Figure 14.45 Intraprotein pores in hyaluronan synthase and cellulose synthase. Top left: “A model for interaction of the HAS [hyaluronan synthase] tandem-motif region with HA [hyaluronan, the #s refer to membrane domains]. The… growing HA-UDP chain, containing alternating GlcNAcβ1,4 (blue squares) and GlcUAβ1,3 (blue-white diamonds) attached to UDP [uridine diphosphate] (red inverted triangle) at the reducing end. Preliminary results indicate that the nonreducing end contains a chitin oligomer cap with four GlcNAc residues, which is the primer on which HA synthesis is initiated” [14.18] (reprinted with permission of Oxford University Press). Top right: AFM experiment suggesting that raphe fibrils are multistranded [14.153] (like cellulose) (reprinted with permission of Elsevier). Bottom: “Updated models of plant CESAs [cellulose synthases] and CSCs [cellulose synthesis complexes]. (a) A computational model of a plant CESA catalytic domain with P-CR and CSR regions (light grey). The glucan chain (purple) is from the homologous Rhodobacter structure. The location of the transmembrane helices (TMH) is represented with grey boxes. (b) Three CESAs, encoded by three different genes, may interact to form a trimeric particle, which in turn may assemble into a hexameric rosette, depicted in (c). The glucan chains are represented in red. (Image (a) is adapted from [14.339] and is courtesy of Jonathan Davis)…. the intra-protein tunnel [pore]… provides a low-energy pathway for translocating the growing glucan chain to the external membrane surface from the cytoplasmic side, where the catalytic site transfers a glucose residue from UDP-glucose to the reducing end of the glucan” [14.62] (reprinted with permission of Elsevier).Figure 14.46 Left: “Stages in secretion at the raphe (hypothetical). (a) Polysaccharide fibrils are discharged from a vesicle into the raphe at the central pore. The preferred secretory sites are at both central and apical (not shown) raphe endings; (b) Secreted fibrils experience hydration and begin to swell and elongate. A second vesicle is docked at the plasmalemma and primed for exocytosis. (c) After considerable swelling the mucilaginous strands project through the raphe; their proximal ends are attached to the plasmalemma and their distal ends are free to make contact with a substratum; (d) Strands, which were produced at the raphe ending, have moved along the raphe but still occupy the slit and maintain attachment to the plasmalemma. Microfilaments act as a barrier preventing vesicle discharge here (not evident in all cells)” [14.93] (reprinted with permission of Elsevier). Note that the hydration of the raphe fibrils does not likely occur within the raphe, as shown, because the walls of the raphe are hydrophobic [14.92] [14.93] [14.122]. On the contrary, hydration at the external tip would generate a force pulling the fibrils out of the raphe. Right: The silica lining the raphe differs from that of the valve [14.66] (reprinted with permission of John Wiley & Sons).Figure 14.47 A general model for noncellulosic polysaccharide biosynthesis [14.362] (reprinted with permission of Oxford University Press).Figure 14.48 “TEM of thin sections of Amphora veneta . 26: T. S. [thin section] through a perinuclear dictyosome [Golgi]. The dictyosome is composed of 8 cisternae (ct) which are blebbing fibrous (v 1) and smaller granular (v 2) vesicles (× 44,000). 27: Oblique L. S. through the central region. Irregularly shaped fibrous (v 1) and smaller granular vesicles (v 2) are apparent (× 32,400). 28: T. S. part of cell to show frustule-cell-membrane interface (fcm). Large numbers of vesicles (v 4) are present outside the cell membrane (cm) but within the silica wall (sw) (× 33,000). 29: T. S. showing fibrous material (fm) extruding from a raphe fissure (rf) (× 22,400)” [14.70] (reprinted with permission of Springer Nature). Note: “The largest [vesicles] (27), were derived from the mature dictyosome [Golgi] cisternae, and were often irregular in outline whilst containing fibrillar material. These vesicles were widely distributed throughout the cytoplasm and the contents were similar in morphology to the extracellular mucilaginous material (29).”Figure 14.49 “Four possible configurations of the microfilament bundles, assuming that they are capable of sliding a short distance relative to the silica raphe. Only the ends of the raphe are shown here, represented by two pores. The view is from the inside of the cell, looking out. The placement of the raphe pores on opposite sides of the apical axis [14.34] is a common feature of raphid diatoms. In this scheme, the microfilament bundles block access of the crystalloid bodies to the raphe along their whole length. In the capillarity model, such blockage would explain the direction and reversal of motion, as indicated on the right” [14.117]. (Reprinted with permission of Elsevier).Figure 14.50 Top: Swirling, draining patterns on a bubble film draining downwards [14.250] (reprinted with kind permission of Michael Reese Much FRMS). Bottom: One frame from a movie showing how dynamic these patterns are [14.288]. The interference colors are due to varying thickness. Note that the flows organize themselves along narrow lines which can draw in material from a wide area. This could be analogous to a diatom’s raphe drawing the liquid cell membrane towards it, bearing proteins (raphan synthase) ready to secrete their polysaccharide raphe fibrils (raphan) upon reaching the raphe.Figure 14.51 Surfing diatoms, Achnanthes and Podocystis [14.95].Figure 14.52 Membrane surfing: A new working hypothesis for the diatom motor (2020). Surfing working model for diatom motility. Raphan synthase is a hypothesized membrane protein that is functional either in the cell membrane, or more likely, in vesicles (crystalloid bodies) into which it deposits raphan, the presumed polysaccharide constituting the raphe fibrils seen in raphes. The whole cell membrane flows, bringing the raphan synthases or the crystalloid bodies, represented as miniatures of Figure 14.51, to the raphe. Myosin motor molecules move along the microfilament bundles (only one shown) hydrodynamically inducing flow of the cell membrane by carrying the vesicles over the raphe. As the vesicles fuse with the cell membrane, they dump their hydrophobic contents into the raphe, where the hydrophobic raphan is shown as short, vertical lines. As the raphe lining is hydrophobic, they fill the raphe via capillarity. Those that come in contact with a substrate, such as the chondrite [14.125] shown here, hydrate, swell, and stick to it. As these hydrated raphan molecules exit, more hydrophobic raphan molecules fill in the raphe, causing a net flow of the anhydrous raphan within the raphe, shown by the small arrow. The result is that the diatom moves relative to the chondrite in the opposite direction, shown by the large arrow. On a flat substrate, the swollen raphan fibrils are left behind as the diatom trail. The flow of the cell membrane determines the direction in which the diatom moves relative to its substrate. Capillarity provides the tremendous force. “On Earth, capillary forces have to fight gravity. But in space, the only resistance is the viscosity of the liquid, which slows the flow but cannot stop it” [14.240]. At the size scale of the diatom raphe, gravity has negligible effect, so the slogan applies.Figure A14.1 Could squeezing out of raphe fluid cause diatom motility? Photo of caulking gun [A.14.4] superimposed with a Cymatopleura diatom [A.14.1] under the Creative Commons Attribution License (CC BY 4.0).
Интервал:
Закладка:
Похожие книги на «Diatom Gliding Motility»
Представляем Вашему вниманию похожие книги на «Diatom Gliding Motility» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Diatom Gliding Motility» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












