Pinnularia floating horizontally on the surface are almost completely enclosed by water. A deformation of the water surface is not visible in phase contrast. There is also no formation of regular patterns due to an attractive interaction. Nevertheless, I consider a slight hydrophobicity to be possible.
Bacteria can often be found on the water. If these form a coherent turf, this probably has a significant influence on the movement of the diatoms. Also, with these observations the water surface showed only a low bacterial density, so that I consider its influence negligible.
When looking vertically at the water surface, the diatoms appear on the water surface in both valve view and girdle band view, with the valve view dominating. Occasionally there is a 90° rotation around the apical axis and thus a change between the two views. Presumably an activity of the raphe in the area of the helictoglossa is responsible for it. As on substrate, Pinnularia in valve view have a high mobility and cover longer distances, while in girdle band view back and forth movements are carried out. The movement is very similar to that on a solid substrate. Occasionally Pinnularia in valve view show rotations around the pervalvarous axis, which cannot be found on substrate. The adhesion to the substrate will probably prevent such rotations. In practice, this movement is often accompanied by a drift movement. In this context, it should be noted that the collective movement of two Pinnularia also appeared. I suspect the coupling by adhering EPS lumps.
The observations of Pinnularia gentilis , which move actively on the water surface, whereby the driving raphe is completely below the water surface, again support Bertrand’s [1.4] hypothesis of a wavelike movement of microfibrils.
1.6 Formation of Flat Colonies in C ymbella lanceolata
Cymbella species either are tube dwelling, develop stalks that often branch into tree-like structures, form colonies directly on substrates or are free-living [1.28]. The transition between free-living and colony-forming is smooth, as diatoms often leave colonies and develop new ones elsewhere. This is the topic of the observations described subsequently.
First, Cymbella lanceolata with a length of approx. 190 μm is discussed ( Figure 1.32). The adhesive EPS excretions are clearly visible in phase contrast, DIC or PlasDIC ( Figure 1.33).
A longer observation of accumulations reveals these processes:
1 1. Diatoms detach from a colony and move away from the colony. This typically happens at the edge of accumulations.
2 2. Diatoms move within the space between the colonies.
3 3. Diatoms meet an existing colony and remain in this cluster.
4 4. Diatoms stop their movement and attach themselves to the substrate.
5 5. Diatoms reproduce asexually inside and outside colonies.
Short-term contacts between diatoms are not mentioned here, as they are transient and of minor importance for the establishment of structures. The relevant steps for the structure formation are exemplarily illustrated in Figure 1.34. Diatoms that attach to colonies usually remain on the edge of the colony. As they themselves secrete EPS, the area produced by EPS deposits is continuously increasing. Events 1, 2 and 3 do not allow the emergence of new colonies. A colony can develop from individual adherent diatoms according to 4 by following cell divisions and attachment of diatoms.

Figure 1.32 Cymbella lanceolata .

Figure 1.33 Two small colonies photographed with PlasDIC.

Figure 1.34 Elementary steps that contribute to structure formation.
To give an impression of the movement activity of the freely moving diatoms, images were taken in artificial daylight for over one hour (one picture every 10 s) and superimposed ( Figure 1.35). The movement activity of diatoms requires sufficient light intensity. With increasing intensity, the raphes become active in the observed Cymbella , regardless of whether they move freely or are in a colony. As a result, more and more diatoms detach from colonies. The driving force then exceeds the adhesion to the substrate caused by EPS. For demonstration, two small colonies were irradiated with a light intensity between 7000 lx and 9000 lx. This is significantly above the intensities used in cultivation, which were at about 500 lx. The microscope illumination with a color temperature of approx. 3000 K was used for irradiation. The light intensity at the location of the diatom under observation is essential for the magnitude of the driving force. A high degree of homogeneity of the illumination in the area under observation is not required for this observation. Figure 1.36shows the colonies at the beginning of irradiation and after about two hours. A considerable reduction in size can be recognized.
The removal of diatoms from a colony and migration require sufficient light intensity. Correspondingly, the activity of the movement decreases when the intensity becomes low. When diatoms encounter existing colonies or deposits of EPS on the substrate at low brightness, they adhere to the colonies because they cannot overcome the adhesion (process 3). At very low light intensity or darkness, the free movement comes to rest. The diatoms then excrete an EPS pad, which they use to adhere to the substrate. At light intensities, as utilized in cultivation, the effect of colony reduction is less than demonstrated in Figure 1.36, but still clearly recognizable. For Figure 1.37a culture in light and dark phase was photographed with a daytime cycle of about 12.5 hours of light per day. To take pictures in the dark phase, the culture was illuminated with low intensity from below through a diffusing screen with a white LED. The light intensity in the bright phase was about 200 lx in the dark phase 15 lx. Probably because of this remaining brightness the movement never comes to rest completely. It is striking that all diatoms moving during the day have reconnected to a colony with a few exceptions.

Figure 1.35 Movement activity of diatoms between colonies.
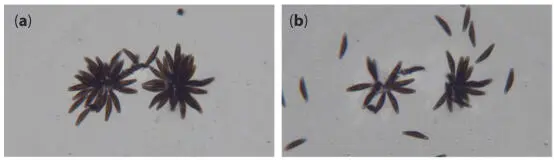
Figure 1.36 Colonies at the beginning of intensive light irradiation (a) and after about two hours (b).

Figure 1.37 Cymbella culture in the light phase (a) and dark phase (b).
To qualitatively and quantitatively study the formation of colonies and the daytime variations of diatoms in and outside the colonies, a culture was observed over 24 days from the time of inoculation, with lighting conditions corresponding to those just mentioned. Another cell line with a diatom size slightly larger than 100 μm was used. Pictures were taken every 10 seconds. The visible area amounted to 8.27 mm × 6.21 mm, corresponding to 2.6% of the cultivated area.
Читать дальше


















