In the textile industry, most dyes are used for fabric coloring, especially synthetic fabrics such as polyester, polyamide, and other polymers. In this case, dyes may impact both the environment and consumers because some dyes cause allergic reactions and skin and respiratory tract irritation. When dyes are inhaled, they attack the immune system and may cause itchy, blocked noses, sneezing, and sore eyes. Furthermore, textile dyes are usually made of azo compounds, which are strong and complex structures made of nitrogen‐nitrogen bonds that can be harmful even at concentrations as low as 1 part per million (ppm). To destroy such components, advanced oxidation processes (AOPs) have shown promising results, despite their high cost and sophisticated equipment requirements.
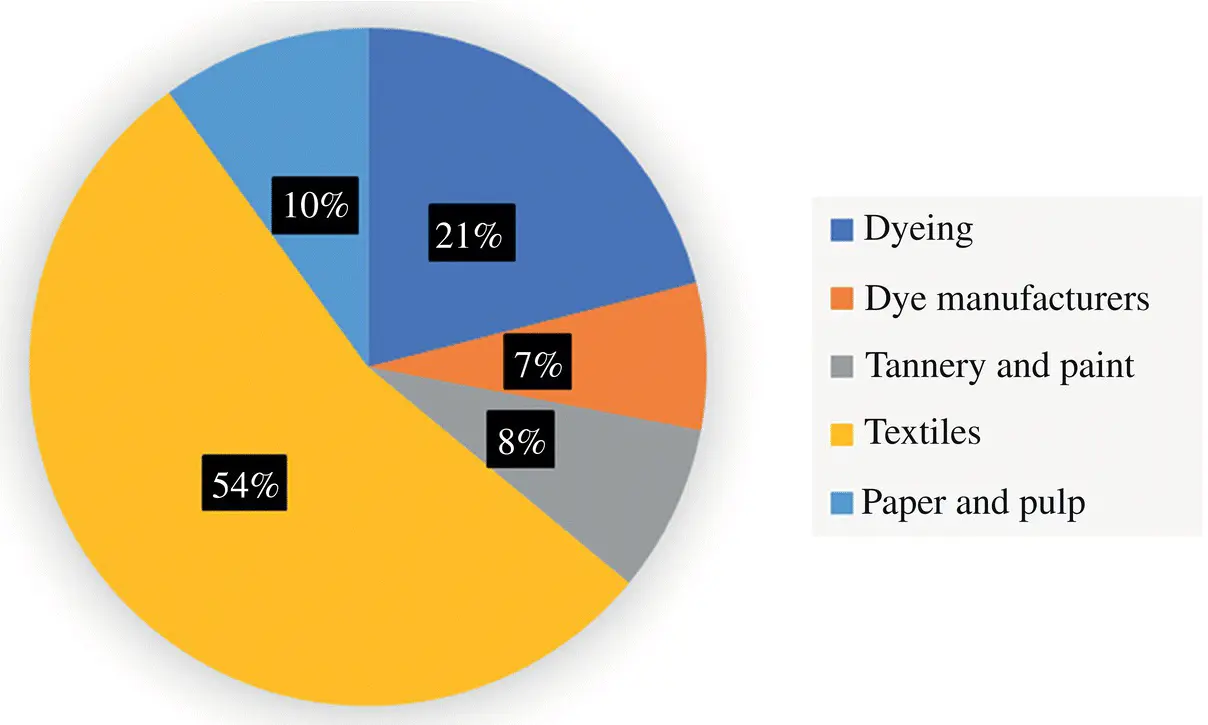
Figure 1.1 Main industries dealing with dyes.
Source: Modified from Katheresan et al., 2018.
In the food industry, dyes are divided into two groups: those that require certification and those that do not. Based on the previous explanation, synthetic dyes such as azo, xanthene, triphenylmethane, and indigoid dyes are subject to a certification process (Barrows et al., 2014) due to their greater risk of toxicity. On the other hand, natural‐based dyes commonly made from plants, insects, or minerals may be exempt from licensing, but their usage still needs concentration limits and attendance to levels of purity. It is worth noting that such licensing practices vary from country to country; hence, deeper understanding is necessary for any individual case. Besides the usual adverse effects listed so far, in rare cases, consuming colored foods may trigger anaphylactic shock that can cause death in minutes if proper treatment is not received, due to hypersensitivity to a dye. Any harmful effect of dye‐containing food may occur due to an individual’s intolerance or overdose of such chemicals when the food was not carefully manufactured.
In a completely different approach from how they are usually applied, dyes can be used with drugs in the pharmaceutical industry for the same purpose. Dyes are gaining credence in therapy due to their ability to engage with drug‐resistant organisms, creating intolerable toxicity for microbes but not humans. A well‐known example is Prontosil Rubrum® from Bayer (IG Farben Conglomerate, Germany), which behaves like a pro‐drug since its reduction products are highly effective against bacterial illness, as shown in Figure 1.2. Consequently, pharmaceutical wastewaters contain, among a variety of complex, toxic, and recalcitrant compounds, dye waste from manufacturing processes and must be treated adequately before discharge.
There is a lack of complete understanding about the most effective technique for removing dyes from wastewater, and some approaches can generate by‐products of secondary pollution. One of the first methods was an activated sludge process. However, although this biological process is relatively cheap and widely implemented worldwide, it is insufficient to remove such hazardous compounds (Katheresan et al., 2018). Another biological method that has achieved better removal is adsorption in biomass simultaneously with biodegradation by enzymes. Further research must take place since enzyme degradation seems to be a cheaper and safer method for dye wastewater treatment (Katheresan et al., 2018).
More expensive methods are available to treat dye wastewater using chemical and physical processes. Among the chemical methods are AOP, electrochemical destruction, ozone oxidation, ozone plus ultraviolet (UV) irradiation, etc. Unfortunately, such methods remain unattractive due to their higher investment and operational costs. On the flip side, physical methods are straightforward approaches relying on mass‐transfer phenomena. These methods encompass adsorption, precipitation, cementation, coagulation, filtration, ion exchange, and reverse osmosis. Such technologies also have advantages and disadvantages and must be carefully analyzed before implementation.
After this brief review of dye wastewater and its hazardous impacts, it should be noted that the structure of dyes turns them into significant threats to the environment. With hydrophilic groups and properties that allow them to add or change color, they can seriously modify the environment and affect all dynamics of ecosystems. In light of this, every industry using dyes during manufacturing processes must be aware of their negative environmental impacts and properly treat wastewater before discharging it into water course.
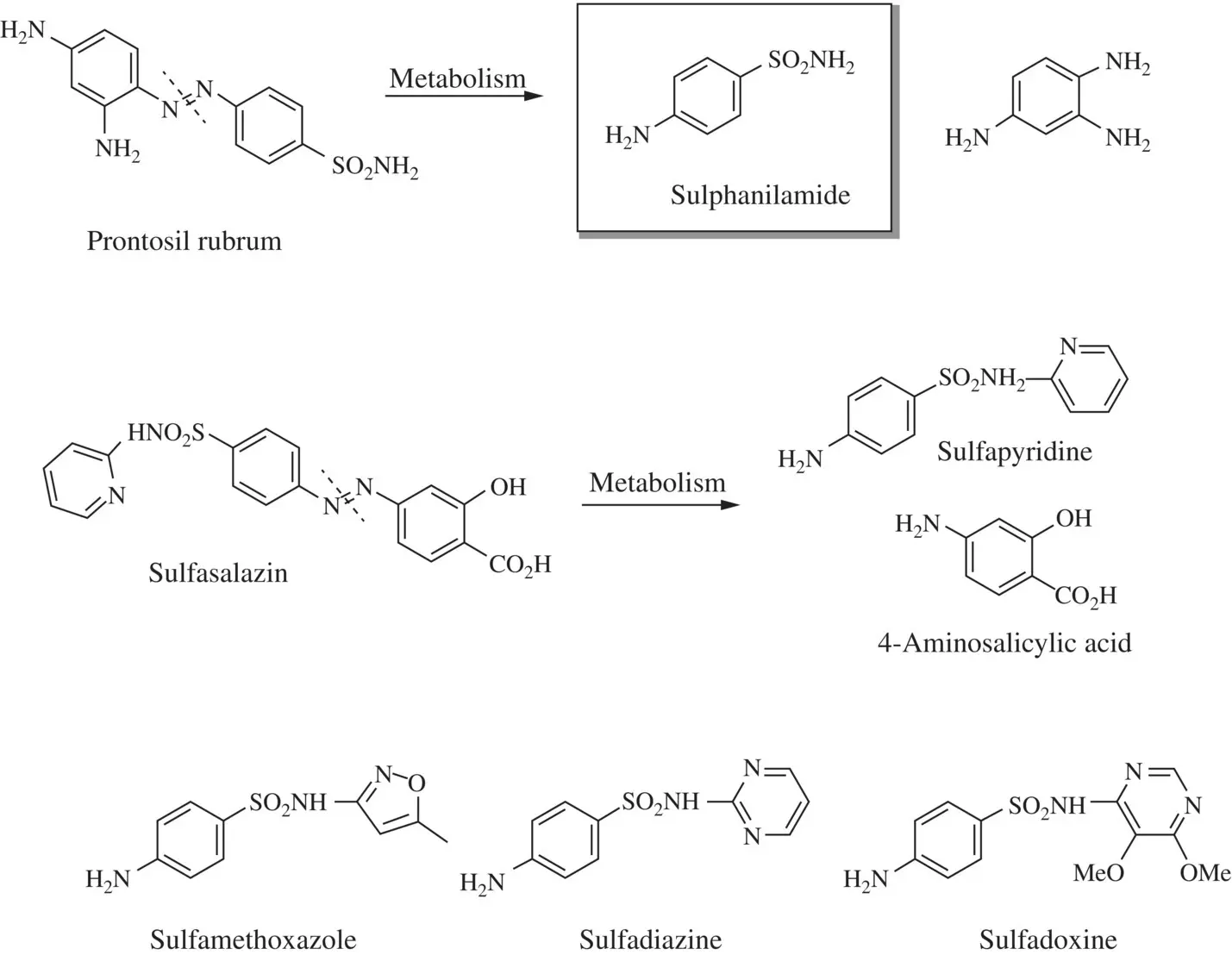
Figure 1.2 Prontosil and its reduction products.
Source: Wainwright, 2008.
Oil and grease (O&G) form a well‐known group of wastewater contaminants including oils, waxes, grease, fats, and other compounds. Their hydrophobic property, together with lower densities compared to water, allows them to form a top layer in bodies of water that interferes with the ecosystem. Moreover, some substances in this pollutant category were found to be plant and animal growth inhibitors and may also cause mutations and cancers among exposed individuals (Odeigah et al., 1997). Similar to dye wastewater, oily wastewater normally has high COD due to its source of generation.
At first, the oily top layer may seem to be a consequence of the density difference and has only an aesthetic or recreational impact. However, this sign of contamination hinders oxygen transfer between the atmosphere and the water body, compromising the dissolved oxygen levels and, therefore, all aquatic life. Besides that, to degrade such compounds, many bacteria consume the available oxygen during their metabolization, further increasing the impact (Ekpu, 1995). If the oil comes from the petroleum industry, it may also be inflammable and contain toxic metal and polycyclic aromatic hydrocarbons (PAHs). O&G also increase the water temperature due to their capacity to absorb solar radiation.
O&G directly and indirectly affect humans. Dermal exposure to oily wastewater can cause skin irritation and strong allergic reactions; when ingested, O&G accumulate in the liver and may cause vomiting, diarrhea, etc. But they also act indirectly, with adverse effects in ecosystems that compromise the basis of the food chain, starting from intoxicating plankton and phytoplankton and contributing to an imbalance that finally reaches the human diet. Furthermore, oily water may enter wastewater treatment plants (WWTPs) and damage pumping systems due to its viscosity, which leads to treatment failure and water and sanitation problems (Diphare et al., 2013).
Over the years, this type of contaminant is increasing in wastewater streams because of greater demand in the industrial sector and population growth. O&G are present in wastewaters from crude oil production, refineries, petrochemical industries, lubricant and cooling materials, compressor condensate, and metal processing. Domestic sewage is also a source of oily substances related to the human diet, restaurants, and car washing.
Oil in wastewater can be classified as a free oil phase, oil‐water mixture, or emulsified oil mixture. As a free oil phase, droplets of oil must have a size greater than or equal to 150 microns; in oil‐water mixtures, the size of oil droplets ranges from 20 to 150 microns; and in emulsified mixtures, their size is smaller than 20 microns. When the droplets are smaller than 5 microns, the oil is considered solubilized (Alade et al., 2011).
Читать дальше














