1 ...6 7 8 10 11 12 ...30 Recent evidence further suggested that, at least in a mouse model, the interval between autograft harvesting and transplantation affected its viability and bone-forming capacity. 119Immediately after autograft harvesting, apoptotic cells were barely detectable, but already within 5 minutes the number of apoptotic cells had nearly tripled. 119The time between harvesting and transplantation also affected the osteogenic potential of an autograft upon transplantation. 119Overall, autografts resemble the osteogenic potential of tissue engineering constructs, showing ectopic bone formation; however, when in small-sized constructs, only around 20 to 70 mm 3in mice 79and in goats. 76Importantly, however, the orthotopic transplantation could not reveal any benefit of cell-based therapies with respect to bone formation at the defect site. 76Thus, beside the fact that osteogenic cells can basically survive transplantation and are a source of osteoblasts at ectopic sites, the overall contribution of the transplanted cells to graft consolidation is yet to be investigated. Nevertheless, our biopsies suggest that the new bone originates from the transplanted bone chips, with new, nascent bone bridging the space between the transplanted bone particles ( Fig 1-12).
1.7 Osteoinductive properties of autografts
The postulated osteoinductive effect attributed to autografts is questionable. By definition, the unlimited osteoinductive effect of autografts can only be proven by ectopic bone formation outside the skeletal system, and not only in or on the bone. Osteoinductive refers to demineralized bone but also dentin matrix, both of which can trigger new bone formation after implantation, e.g. in the muscle of a rat. 63This demineralized bone matrix ultimately leads to the isolation and molecular characterization of bone morphogenetic proteins (BMPs). It is not widely known that the isolation of BMPs requires 5 to 20 kg of bone to obtain sufficient protein for purification testing, including in vitro osteogenic differentiation and in vivo osteoinductive bone formation. 8,83,133However, there is no evidence for ectopic bone regeneration after transplantation of bone chips in a muscle; in fact, it is the opposite – that the bone chips are resorbed without the induction of new bone by host-derived induced osteoblasts. 13
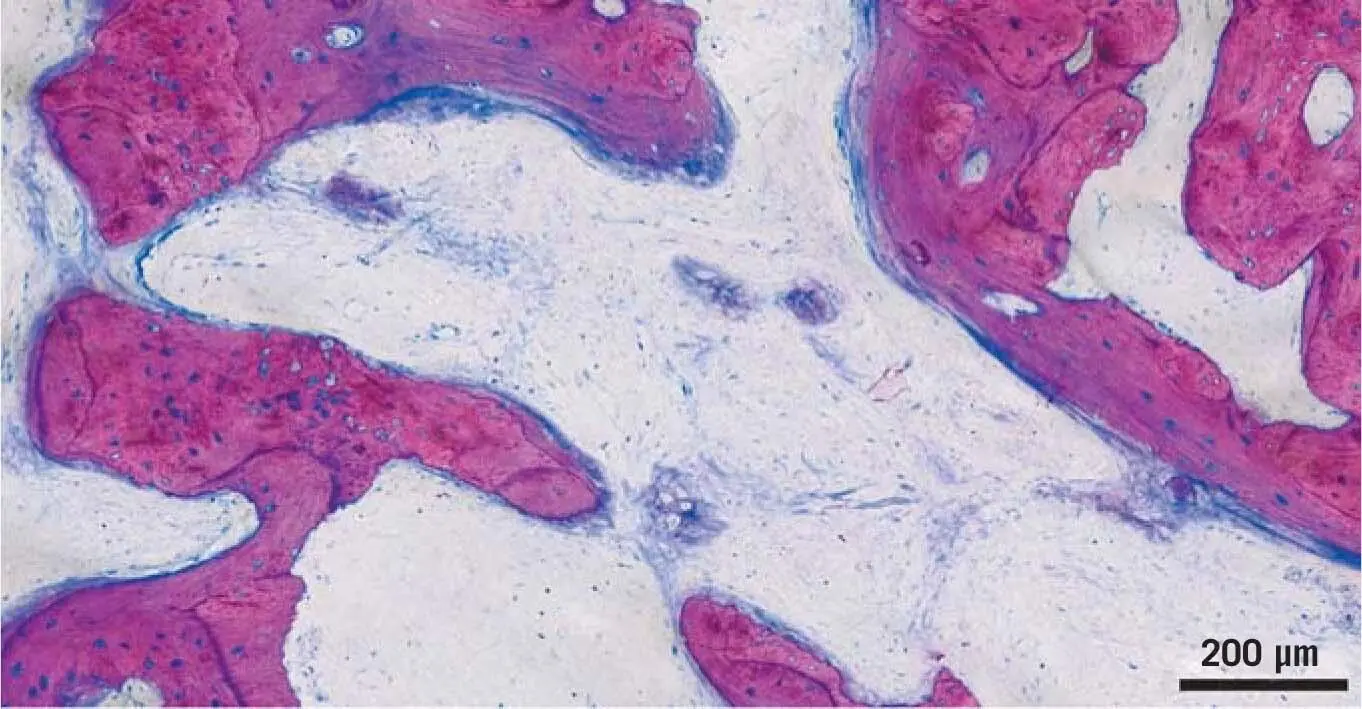
Fig 1-12New bone formation on the surface of transplanted bone chips. In the SBB technique, after 3 months, healing areas of nascent bone formation are detectable. The structure of future bone formation can already be anticipated. Even though the origin of the bone is not defined, it seems that bone formation originates from the transplanted osteogenic cells.
When implanted into the muscle pouches of beagle dogs, bone grafts resorbed quickly, while alloplasts and a synthetic biphasic calcium phosphate showed minor signs of ectopic bone formation. 87Also, in Wistar rats, autologous bone chips from a corticocancellous bone block grafted in a muscle were entirely resorbed after 6 weeks. 88If native bone chips were actually unlimitedly osteoinductive, it would mean that, were bone chips to enter the soft tissue, new bone would be formed there. This side effect of ectopic bone formation in soft tissue would be clinically undesirable. However, there is evidence that, at least during bone remodeling, osteoclasts release TGF-βb1 from the bone matrix, which recruits mesenchymal progenitors to the remodeling sites. 32It was recently confirmed that TGF-β1 released by acid lysis of bone is a major regulator of gene expression in mesenchymal cells in vitro. 117Moreover, acid bone lysates delayed bone formation in a rat calvaria defect model. 116Since the accumulation of these results makes a major involvement of BMPs in graft consolidation unlikely, and proposes that TGF-β1 supports the immigration of progenitor cells, the idea of the osteoinductive properties of autografts needs to revisited, and has to be limited only to the support of bone formation in direct contact with bone tissue.
In summary, the surgical approaches outlined in Chapter 4of this book – the bony lid technique, the SBB technique, and the bone core technique 67,68– nicely reflect the basic principles of successful bone healing introduced in this chapter, which are that:
1. Autografts have favorable osteoconductive properties, allowing bone to form on the surface.
2. Once harvested and used immediately, autografts can exert osteogenic properties where the transplanted cells might contribute to bone formation.
3. The osteoinductive properties of autografts are questionable, and it is desirable to avoid ectopic bone formation at soft tissue sites.
Nevertheless, the resorption of autografts releases growth factors that might support graft consolidation. The amount of resorption can be clinically controlled under certain indications, including the biologic approaches presented in this book.
The prerequisites for successful graft consolidation are mechanically stable conditions in a well-vascularized area with vital bone walls as the origin of the newly formed bone. The definitions of a gold standard should mainly be seen clinically, and defined for each indication, since experimental animal research is mostly based on a short observation time of only a few weeks. It therefore seems worthwhile to resume experimental work on the autogenous bone experiments of the pioneers, ultimately in search of molecular and cellular explanations for the definition of a gold standard.
The support of Dr Stefan Tangl and Toni Dobsak (Core Facility Hard Tissue and Biomaterial Research, Karl Donath Laboratory) in generating the figures for this chapter is highly appreciated.
1. Aghvami M, Brunski JB, Serdar Tulu U, Chen CH, Helms JA. A thermal and biological analysis of bone drilling. J Biomech Eng 2018:140: 1010101–010108. doi: 10.1115/1.4040312.
2. Albee FH. The various uses of the bone graft. Proc R Soc Med 1930;23:855–860.
3. Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001; 10(suppl 2):S96–S101.
4. Alzahrani MM, Rauch F, Hamdy RC. Does sclerostin depletion stimulate fracture healing in a mouse model? Clin Orthop Relat Res 2016;474:1294–1302.
5. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 2005;32:212–218.
6. Baht GS, Silkstone D, Nadesan P, Whetstone H, Alman BA. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J Orthop Res 2014;32:581–586.
7. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013;19:179–192.
8. Bentz H, Nathan RM, Rosen DM, et al. Purification and characterization of a unique osteoinductive factor from bovine bone. J Biol Chem 1989;264:20805–20810.
9. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res 2003;14:251–262.
10. Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011;26:229–238.
11. Botticelli D, Berglundh T, Buser D, Lindhe J. The jumping distance revisited: an experimental study in the dog. Clin Oral Implants Res 2003;14:35–42.
12. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337–342.
13. Boynton E, Aubin J, Gross A, Hozumi N, Sandhu J. Human osteoblasts survive and deposit new bone when human bone is implanted in SCID mouse. Bone 1996;18:321–326.
Читать дальше