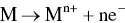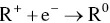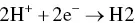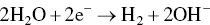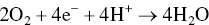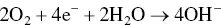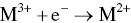Organic Corrosion Inhibitors
Здесь есть возможность читать онлайн «Organic Corrosion Inhibitors» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Organic Corrosion Inhibitors
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
Organic Corrosion Inhibitors: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Organic Corrosion Inhibitors»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
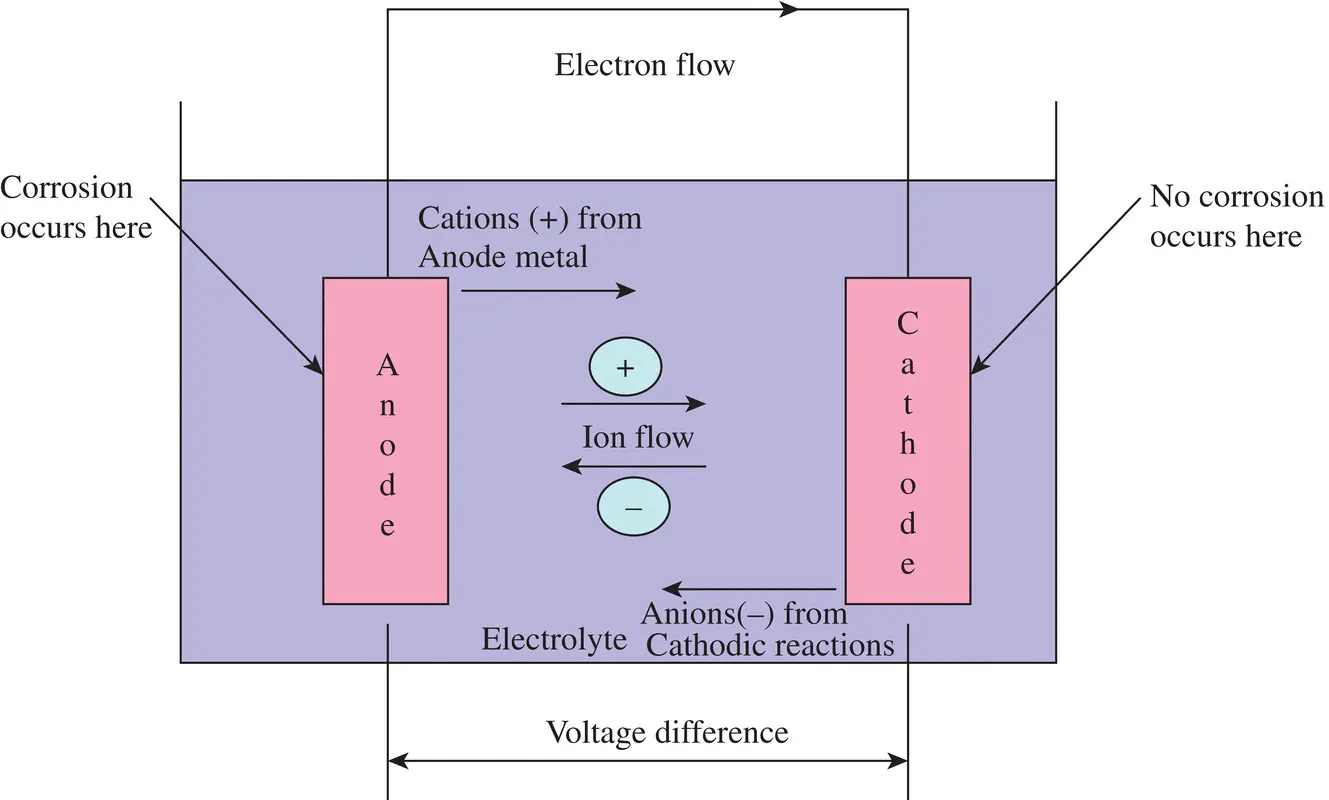
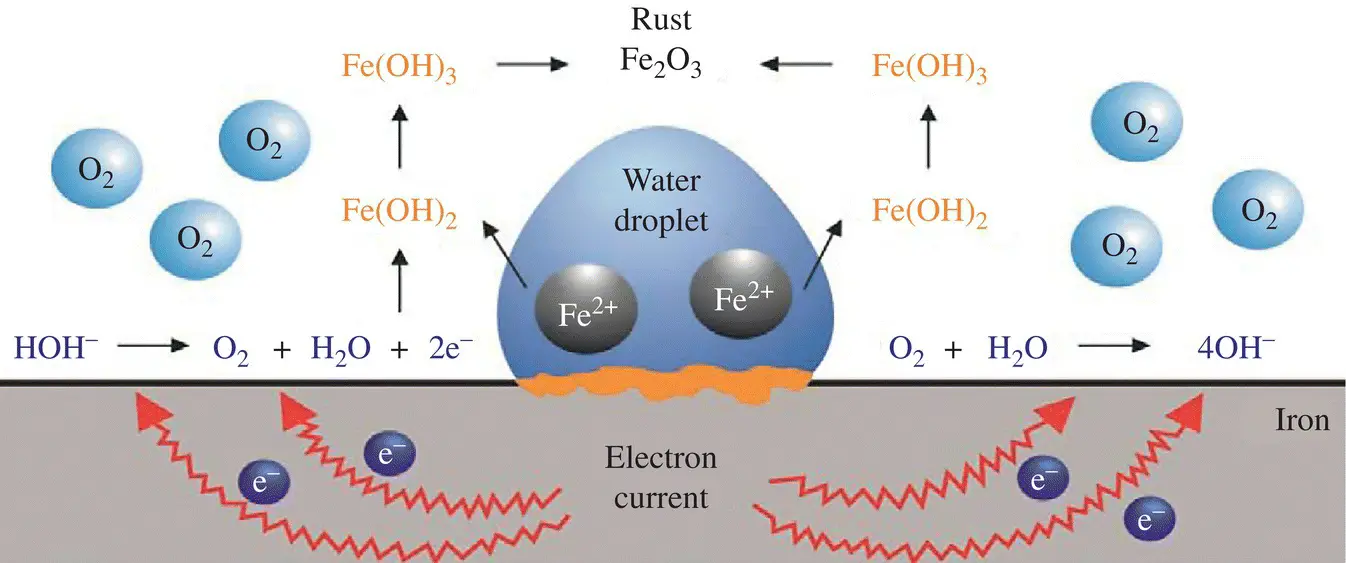
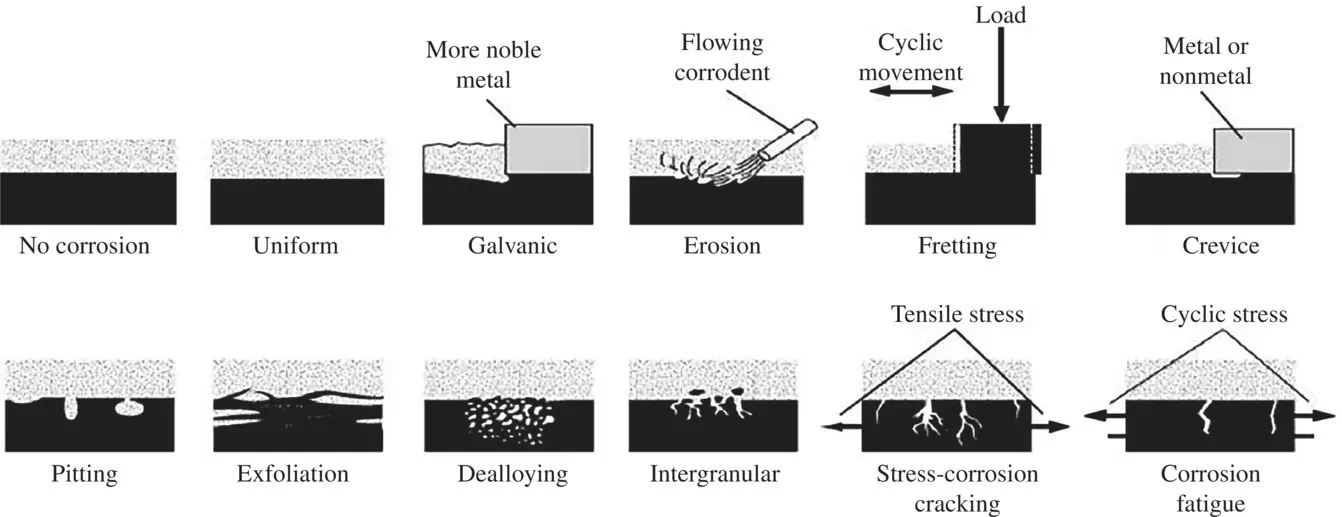
provides up-to-date coverage of all aspects of organic corrosion inhibitors, including their fundamental characteristics, synthesis, characterization, inhibition mechanism, and industrial applications.
Divided into five sections, the text first covers the basics of corrosion and prevention, experimental and computational testing, and the differences between organic and inorganic corrosion inhibitors. The next section describes various heterocyclic and non-heterocyclic corrosion inhibitors, followed by discussion of the corrosion inhibition characteristics of carbohydrates, amino acids, and other organic green corrosion inhibitors. The final two sections examine the corrosion inhibition properties of carbon nanotubes and graphene oxide, and review the application of natural and synthetic polymers as corrosion inhibitors. Featuring contributions by leading researchers and scientists from academia and industry, this authoritative volume:
Discusses the latest developments and issues in the area of corrosion inhibition, including manufacturing challenges and new industrial applications Explores the development and implementation of environmentally-friendly alternatives to traditional toxic corrosion inhibitors Covers both established and emerging classes of corrosion inhibitors as well as future research directions Describes the anticorrosive mechanisms and effects of acyclic, cyclic, natural, and synthetic corrosion inhibitors Offering an interdisciplinary approach to the subject,
is essential reading for chemists, chemical engineers, researchers, industry professionals, and advanced students working in fields such as corrosion inhibitors, corrosion engineering, materials science, and applied chemistry.