Fundamentals of Aquatic Veterinary Medicine
Здесь есть возможность читать онлайн «Fundamentals of Aquatic Veterinary Medicine» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Fundamentals of Aquatic Veterinary Medicine
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Fundamentals of Aquatic Veterinary Medicine: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Fundamentals of Aquatic Veterinary Medicine»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Covers the competencies necessary to assure the highest quality of aquatic veterinary services Fundamentals of Aquatic Veterinary Medicine
Fundamentals of Aquatic Veterinary Medicine — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Fundamentals of Aquatic Veterinary Medicine», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Acidification of our oceans has a great effect on the ecosystem of the ocean. It is leading to changes in the phytoplankton that are available for ingestion by fish and cultivation. It is predicted to decrease the available fish population of our reefs, which will damage the animal protein consumption of many developing nations. The high fluctuations of pH in the estuaries and coastal areas have the potential to greatly affect fisheries. Mitigation of these fluctuations should include consistent monitoring of the water to enable fisheries to add buffering if necessary (Clements and Chopin, 2017). Options for buffering may include:
Crushed shell hash in the sediment
Sodium bicarbonate
Movement of fish pends away from river mouths.
Further mitigation strategies should include breeding plants and animals that have a high tolerance for low pH. Integration of a multitrophic aquaculture system can help to mitigate pH fluctuations and other stress caused by run‐off and increased population. An integrated system should include three levels of nutrient absorption (Clements and Chopin, 2017):
Shell fish: small organic particle suspension extractive.
Seaweed: dissolved nutrients suspension extractive.
Invertebrates: large organic particle deposit extractive.
While acidification of natural aquasystems can only be mitigated by global reduction of atmospheric carbon dioxide, further research is necessary to fully understand the impacts of global climate change and the acidification of our oceans.
1.3.2.14 Ammonia, Nitrite and Nitrate
Ammonia is a colorless, odorless substance which can accumulate in aquasystems and cause direct mortality, a decrease in production and increased incidence of many diseases. In water, ammonia occurs in two forms, which together are called the total ammonia nitrogen. Ammonia gas is usually released from the gills or from decomposing organics dissolved in the water. Some reacts with the water to produce ammonia ions. The remainder is present as un‐ionized, which is acutely toxic to aquatic life. Chemically, these two forms are represented as NH 4 +and NH 3. NH 4 +is called ionized ammonia because it has a positive electrical charge, and NH 3is called un‐ionized ammonia (UIA), since it has no charge. This is important to know, since NH 3, UIA is the form that is toxic to fish. The percentage of UIA in solution depends on the pH and temperature of the water; as both go higher, so does the toxicity.
A typical aquasystem has bacteria, which in the presence of dissolved oxygen converts (oxidizes) ammonia to the intermediate form of nitrite and then to nitrate. Ammonia is converted into nitrite (NO 2 –) due to the influence of Nitrosomonas bacteria. Nitrite (NO 2 –) is converted into nitrate (NO 3 –) due to the influence of Nitrobacter bacteria. Most test kits measure total ammonia nitrogen, so the aquarist must determine what percentage of the total is toxic.
Nitrite is an intermediate in the oxidation of ammonium to nitrate. Nitrite is less toxic to fish than ammonia. An elevated ambient nitrite concentration is a potential problem for freshwater fish since nitrite is actively taken up across the gills in competition with chloride. Nitrite is a well‐known toxicant for fish, as well as a disrupter of multiple physiological functions including ion regulatory, respiratory, cardiovascular, endocrine and excretory processes. One critical consequence of nitrite accumulation is the oxidation of hemoglobin to methemoglobin, compromising blood oxygen transport (Kroupova et al ., 2005). Nitrite toxicity to fish varies considerably and depends on many external and internal factors. Among the most important ones are water quality (e.g. pH, temperature, cation, anion and oxygen concentration), length of exposure, fish species, fish size and age, and individual fish susceptibility.
Nitrate is relatively non‐toxic; however, high concentrations reduce animal growth and can decrease survival. For example, concentrations over about 400 mg NO 3 –N/l have been shown to depress the growth rate and survival of marine shrimp ( Litopenaeus vannamei ). In intensive biofloc aquaculture systems used for shrimp culture, concentrations of 400 mg NO 3 –N/l have been observed after using water for roughly three culture cycles. Salinity influences the toxicity of nitrate, as it does with ammonia and nitrite. At higher salinities, these compounds are less toxic to aquatic animals. Several options exist for dealing with nitrate, including water exchange, phytoremediation, denitrification, and heterotrophic assimilation (Svobodova and Kolarova, 2004; Svobodova et al ., 2005; Tepper, 2000).
It is recommended that un‐ionized ammonia should be less than 0.02 ppm to prevent stress and reduced growth. Ammonia has been reported to be lethal to catfish at about 0.4 ppm (Jensen, 2003). Ammonia, nitrite and nitrate are labile ions and must be analyzed rapidly or samples frozen as soon as possible after filtration. Samples that cannot be submitted within a few hours should be filtered (0.45 mm) then frozen to reduce the loss of ammonia and changes in nitrite/nitrate concentrations. Plain blood collection tubes are suitable containers for freezing. Two 10‐ml tubes will provide enough samples for all three tests. Do not fill completely; leave space for expansion during freezing.
1.3.2.15 The Nitrogen Cycle
In aquaculture, the nitrogen cycle eliminates ammonia from the water column by bioconversion to nitrite and then nitrate. Nitrate is used by plants, including algae, as a nutrient. This constant change from ammonia to nitrite to nitrate is called the nitrogen cycle ( Figure 1.1). In ponds, this process takes place in the surface layers of the mud, but in tanks or aquaria, a special place is provided for the bacteria to live and flourish. This is called a biological filter, or biofilter. One important point to mention about the nitrogen cycle is that both groups of nitrifying bacteria need oxygen to function. If oxygen levels are insufficient, the process can slow down and the levels of ammonia and nitrite will rise in the system.
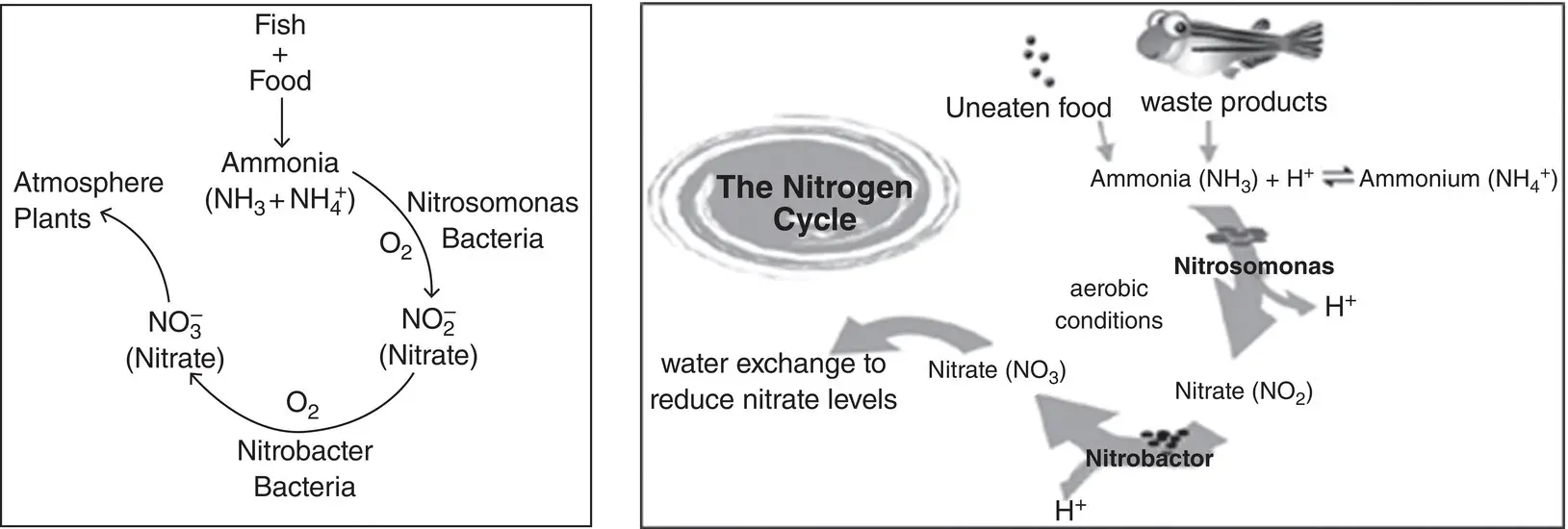
Figure 1.1 The nitrogen cycle (Francis‐Floyd, 2003).
Source: image on the right courtesy of Gillian Taylor.
1.3.2.16 Interaction of Chemical and Physical Properties of Water that Affect Aquatic Animal Health
Some chemicophysical parameters of water have a direct influence upon fish health. Any abrupt or large fluctuations of these parameters often cause a state of stress in fish, sometimes resulting in widespread disease outbreaks. Dissolved oxygen content, pH, turbidity, temperature, introduction of some chemicals, detergents, pesticides and naturally produced toxic compounds like hydrogen sulfide, ammonia, and dinoflagellate toxins are potential stress‐related parameters. Carbon dioxide concentration up to 20–30 mg/l can be tolerated by fish provided that oxygen is near saturation. At lower levels of dissolved oxygen, the toxicity of carbon dioxide increases. The optimum pH range is between 6.7 and 8.6; liming agents may be applied to correct a low pH.
Ammonia concentration above 1 mg/l can indicates organic pollution. Hydrogen sulfide toxicity increases with decreasing pH and it is harmful even at a concentration of 1 mg/l. Making the aquasystem environment more congenial and hygienic reduces stress and promotes fish health. For example, excessive application of inorganic fertilizers and accumulation of organic matter in older aquasystems may cause an over production of phytoplankton, and the appearance of algal and bacterial blooms, leading to dissolved oxygen depletion to lethal levels. For health and optimum growth, the dissolved oxygen level should not drop below 2–5 mg/l. Carbon dioxide concentration up to 20–30 mg/l can be tolerated by fish provided oxygen is near saturation. Nephrocalcinosis in salmonids has long been recognized as a pathological entity related to high dissolved CO 2, eventually leading to the formation of large mineralized deposits within the excretory tissue of the kidney and associated kidney pathology. The condition can result in poor condition and performance and occasional fish loss, particularly if other stressors are present.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Fundamentals of Aquatic Veterinary Medicine»
Представляем Вашему вниманию похожие книги на «Fundamentals of Aquatic Veterinary Medicine» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Fundamentals of Aquatic Veterinary Medicine» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.